Summary
The authors found that coronary thrombosis in patients undergoing thrombectomy because of ST-elevation myocardial infarction with concomitant COVID-19 is more severe. In these patients percutaneous coronary intervention less frequently ends in an optimal result. COVID-19 is an independent and strong predictor of patient qualification for aspiration thrombectomy in ST-elevation myocardial infarction.
Introduction
Initially, with SARS-CoV-2 coronavirus infections, the main focus was placed on the infectious symptoms and the pulmonary complications that could eventually lead to progression of respiratory failure and, in many cases, inevitably lead to the patient’s death. It was quickly recognized that infection with the new coronavirus could generate symptoms from other systems, likely due to the prevalence of the receptor by which the virus enters the host cell. One of the first observations made was the effect of infection on the coagulation system. It was reported that patients with coronavirus disease 2019 (COVID-19) had an increased incidence of complications such as ischemic stroke, pulmonary embolism and acute coronary syndromes [1–3]. It has not been fully clarified what leads to the procoagulant state in COVID-19. Most likely, there are complex mechanisms between the innate immune response, coagulation and fibrinolysis pathways and vascular endothelial damage, as well as individual variables including mutations of factor V Leiden and prothrombin G20201A [4, 5]. The pathology observed in the course of COVID-19 in a mouse model was similar to that observed in patients with severe COVID-19, with evidence of diffuse bronchoalveolar injury with edema, fibrin deposits, leukocyte infiltration and pneumocyte hypertrophy [4]. Also there are significant histopathological changes in the accumulation of T lymphocytes (CD4-CD8 located around blood vessels are visible in the post-mortem examination of COVID-19 patients) [6]. The impact of disruptions of the fibrinolysis system in the course of COVID-19 infection may play a significant role not only in terms of cardiac diseases. The importance of this system and its disorders in the course of infection is also confirmed by the observations of other specialists. A complication that can be observed in patients with severe COVID-19 is pulmonary fibrosis, in which the formation of fibrin in the intraalveolar compartment and proteases of the coagulation system and plasminogen activation (plasminergic) system play an important role, which respectively create and break down fibrin [7]. In a study of the effect of drugs on pulmonary fibrosis in mice, lower fibrinogen levels and higher levels of tPA, the main intravascular activator of fibrinolysis, were found in the treatment group, which correlated with inhibition of fibrosis progression in mice [8]. Experts suggest that therapy to reduce fibrotic lesions used in the early stages of SARS-CoV-2 infection may have significant benefits [9].
It seems that the procoagulant potential in the course of SARS-CoV-2 virus infection may be enhanced in the course of acute coronary syndrome (ACS), where coagulation also occurs. As a result, this additive effect may lead to a more severe course and be associated with a higher risk of death [10, 11].
Aim
The aim of the study is to determine whether and how COVID-19 affects the clinical parameters of ST-elevation myocardial infarction (STEMI) patients undergoing aspiration thrombectomy during percutaneous coronary intervention (PCI) as well as the application and effect of thrombectomy in STEMI patients.
Material and methods
In order to determine the most important factors that, due to the enhanced clotting potential of SARS-CoV2 coronavirus infection, may have affected thrombus formation in the coronary vessel, as well as whether COVID-19 infection alone affected the frequency of aspiration thrombectomy, we analyzed patients with STEMI, based on the National Registry of Invasive Cardiology Procedures (ORPKI) [10].
The study group consisted of patients undergoing invasive treatment for STEMI from March 4, 2020, when the first COVID-19 infection was confirmed in Poland, until March 4, 2022.
The group of patients collected for analysis, namely those who qualified for treatment of STEMI and were registered in ORPKI, included 29915 patients, of whom 3139 underwent aspiration thrombectomy. This group consisted of 2828 COVID-19 negative patients and 311 patients who were diagnosed with COVID-19. The antigen tests were used to confirm positive cases, either in the ambulance or at the destination hospital. Due to the need to adhere to time standards, there was no waiting for PCR test results. Patients with suspected COVID-19 (as recommended for triage by the National Institute of Public Health and the Ministry of Health) were treated as potentially COVID-19 (+). A diagnosis of COVID-19 was always available before any interventional procedure (angiography or percutaneous coronary intervention) and was recorded in the ORPKI online database. Swabs for molecular RT-PCR were always collected before the procedure. The study included cases of patients who underwent primary PCI; rescue and facilitated PCI were excluded. The time from symptoms onset to PCI was less than 24 h.
We carried out a pooled analysis of factors predisposing to aspiration thrombectomy in STEMI patients undergoing invasive treatment. The mentioned patients received acetylsalicylic acid (ASA), P2Y12 or anticoagulation during PCI, which was included in the ORPKI registry; the remaining patients received the drugs earlier, which was not shown in the ORPKI registry. The ORPKI registry does not provide specific information about thrombolysis during PCI. Data on thrombus burden grades were not collected as part of the ORPKI registry. Chronic kidney disease was defined in compliance with the recommendations as the presence of an estimated glomerular filtration rate (eGFR) of less than 60 ml/min/1.73 m². Two groups of patients were compared: patients with STEMI but without confirmed infection (COVID-19(–)) and patients with STEMI with confirmed infection (COVID-19(+)). We carried out multivariable regression analysis of clinical, prehospital and pharmacological factors to the endpoint of aspiration thrombectomy. The authors selected variables with a potential impact on the course of PCI; these were mainly clinical parameters. Patients qualified for invasive treatment signed informed consent forms in accordance with the recommendations of the 1964 Declaration of Helsinki. As we used anonymous data from the ORPKI database, the study did not require approval from the Bioethics Committee.
Statistical analysis
Nominal variables were presented as counts and percentages, whereas continuous variables were presented as means with standard deviation as well as median with the first and the third quartiles. Normality was assessed by Shapiro-Wilk test. Equality of variances was assessed using Levene’s test. Differences between two groups were compared using Student’s or Welch’s t test depending on the equality of variances for normally distributed variables. The Mann-Whitney U test was used for non-normally distributed continuous variables. Comparison of nominal variables between analyzed groups was performed using the Pearson χ2 test or Fisher’s exact test. Multivariable logistic regression models were used to estimate odds ratios with 95% confidence intervals and p-values. A p-value of less than 0.05 was considered significant. No missing data imputation was performed. All available data were analyzed. The final model was run on all cases. The outcome was thrombectomy and exposure was COVID-19. Predictors were age, gender, weight, diabetes, previous myocardial infarction (MI), previous PCI, previous coronary artery bypass grafting (CABG), smoking status, psoriasis, arterial hypertension, kidney disease, chronic obstructive pulmonary disease (COPD), Killip class at admission and cardiac arrest at baseline. Effect modifiers were advanced forms of coronary artery disease (stenosis of left main coronary artery (LMCA), multivessel disease with or without stenosis of LMCA). Confounders were time from pain to first medical contact (FMC) and time from FMC to angiography. All statistical calculations were performed with R version 4.1.3 (R Core Team, Vienna, Austria, 2022).
Results
Among 29915 patients with STEMI included in the study 3139 underwent aspiration thrombectomy. 2828 patients were COVID-19 (–) and 311 patients were COVID-19 (+).
Patients’ distribution is presented in Figure 1. Baseline characteristics of clinical factors showed that patients with STEMI and concomitant COVID-19 who had undergone aspiration thrombectomy were in a higher Killip class (class IV; n = 33 (12.31%) vs. n = 138 (5.84%); p < 0.0001), more frequently had chronic obstructive pulmonary disease (COPD) (n = 66 (2.33%) vs. n = 16 (5.14%); p = 0.003), chronic total occlusion (CTO) (n = 63 (2.23%) vs. n = 13 (4.18%); p = 0.034), had higher weight (n = 82 (73; 95) vs. n = 85 (77; 95); p = 0.026) and longer time from first contact to inflation or angiogram in minutes (75 (55; 120) vs. 90 (58.5; 142.5); p < 0.001) as well as being more likely to experience out-of-hospital cardiac arrest (n = 137 (4.84%) vs. n = 25 (8.04%); p = 0.016) (Table I). Patients with STEMI and concomitant COVID-19 who underwent aspiration thrombectomy during PCI more often received unfractionated heparin (UFH) (n = 2295 (81.15%) vs. n = 268 (86.17%); p = 0.03), acetylsalicylic acid (n = 908 (32.11%) vs. n = 124 (39.87%); p = 0.006), clopidogrel (n = 370 (13.08%) vs. n = 50 (16.08%); p = 0.001) and ticagrelor (n = 843 (29.81%) vs. n = 119 (38.26%); p = 0.001) as well as bivalirudin (n = 20 (0.71%) vs. 6 (1.93%); p = 0.024). Total radiation dose was higher in COVID-19 patients (551 (315; 938) vs. 621 (376.5; 1130.5); p < 0.001) and these patients required thrombolysis during PCI more often (n = 24 (0.85%) vs. n = 9 (2.89%); p < 0.001). Post-PCI Thrombolysis In Myocardial Infarction (TIMI) 3 flow was obtained less frequently (n = 2388 (87.19%) vs. n = 248 (80.52%); p < 0.001) (Table II). Multivariable regression analysis showed that independent predictors of thrombectomy were Killip class IV (OR = 2.06 (1.66–2.55); p < 0.001), COVID-19 (OR = 1.23 (1.05–1.43); p = 0.001), active smoking status (OR = 1.18 (1.07–1.31); p = 0.001), male gender (OR = 1.13 (1.01–1.27); p = 0.032), age (OR = 0.99 (0.98–0.99); p < 0.001), weight (OR = 1.01 (1.002–1.008); p = 0.003), multivessel disease with left main coronary artery (OR = 0.81 (0.74–0.89); p < 0.001) or without LMCA stenosis (OR = 0.63 (0.51–0.77); p < 0.001) and time from first medical contact to angiography (OR = 0.99 (0.9987–0.9996); p = 0.001) (Table III). Factors affecting qualification for thrombectomy are also shown in Figure 2.
Figure 1
Distribution of patients with STEMI treated by aspiration thrombectomy
COVID-19 – coronavirus disease 2019, PCI – percutaneous coronary intervention, STEMI – ST-elevation myocardial infarction, TIMI – Thrombolysis In Myocardial Infarction.
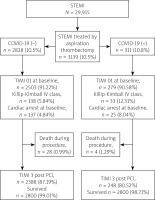
Figure 2
Independent predictors of aspiration thrombectomy in STEMI
COVID-19 – coronavirus disease 2019, FMC – first medical contact, LMCA – stenosis of left main coronary artery, STEMI – ST-elevation myocardial infarction.
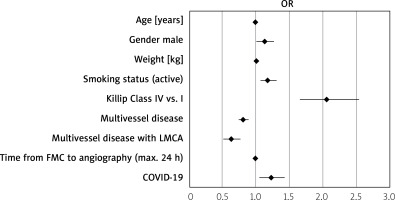
Table I
Baseline characteristics of clinical factors in STEMI patients (n = 29915) undergoing aspiration thrombectomy during PCI
[i] CABG – coronary artery bypass grafting, COPD – chronic obstructive pulmonary disease, COVID-19 – coronavirus disease 2019, CTO – chronic total occlusion, LMCA – left main coronary artery, MI – myocardial infarction, PCI – percutaneous coronary intervention, STEMI – ST-elevation myocardial infarction.
Table II
Descriptive characteristics of procedural factors in STEMI patients undergoing aspiration thrombectomy during PCI
[i] ASA – acetylsalicylic acid, Cx – critical stenosis of circumflex artery, COVID-19 – coronavirus disease 2019, GPI IIb/IIIa – IIb/IIIa glycoprotein inhibitor, LAD – critical stenosis of left anterior descending artery, LIMA-LAD – critical stenosis of left internal mammary artery – left anterior descending bypass, LMCA – critical stenosis of left main coronary artery, LMWH – low molecular weight heparin, PCI – percutaneous coronary intervention, RCA – critical stenosis of right coronary artery, STEMI – ST-elevation myocardial infarction, SVG – critical stenosis of saphenous vein graft, TIMI – Thrombolysis In Myocardial Infarction, UFH – unfractionated heparin.
Table III
Clinical factors influencing the use of aspiration thrombectomy in all patients
[i] CABG – coronary artery bypass grafting, COPD – chronic obstructive pulmonary disease, COVID-19 – coronavirus disease 2019, CTO – chronic total occlusion, FMC – first medical contact, LMCA – critical stenosis of left main coronary artery, MI – myocardial infarction, PCI – percutaneous coronary intervention, STEMI – ST-elevation myocardial infarction.
Discussion
In the course of COVID-19 infection, an increased incidence of thromboembolic complications was very quickly noted, and disorders of the hemostasis system became distinguished from the previously known coagulopathy, which was referred to as COVID-19-associated coagulopathy (CAC). Equally quickly, a different course of STEMI in the course of SARS-CoV2 infection began to be observed. It was found that some of the infected patients undergoing invasive examination did not have an identifiable coronary lesion [12], and the occurrence of non-obstructive coronary artery disease was significantly more frequently observed in COVID-19 STEMI than in the control group (13% vs. 1%, p = 0.03) [13]. In addition, there have been reports that this problem occurred with an increased frequency in women (1/3 of women vs. 1/5 of men) [14]. Information is available in the literature on cases of patients who developed coronary artery embolism during COVID-19 infection. During angiography, thrombectomy was performed, and due to the absence of a coronary lesion, stent implantation was abandoned, and then anticoagulant therapy was continued with a satisfactory effect [15]. Similar clinical cases have also been reported by other authors [16]. The angiographic image in the form of extensive and multivessel thrombosis, regardless of the presence of atherosclerotic plaques, poses a new therapeutic challenge and may be associated with an increase in the incidence of stent thrombosis [17], which in in-hospital settings may increase up to five times [18]. In a large multicenter, retrospective study in patients with COVID-19 and STEMI disease, the percentage of stent thrombosis in patients undergoing PCI treatment was estimated to be 21% vs. 1% compared to the control group [19]. Interesting conclusions were provided by the study of Cornelissen et al., who assessed the inflammatory potential and thrombogenicity of different stents using the blood of healthy volunteers, enriched with high levels of IL-6 and TNF-α to simulate a cytokine storm, similar to that occurring in the course of COVID-19. The study indicates that patients with SARS-COV2 infection should be treated with stents with the lowest thrombogenicity, using nanocoatings that prevent platelet adhesion [20]. The prevention of stent thrombosis is also influenced by other variables that have already been studied. Preference is given to stents made of materials that reduce adhesion and activation of platelets through albumin adsorption. Of great importance after stent implantation is the rate of endothelial coverage, which is influenced by factors such as thinner struts, type of polymer, type of drug and its quantity [21].
Our results indicate that COVID-19 infection is a strong independent predictor of a patient’s eligibility for aspiration thrombectomy in the course of STEMI. Similar conclusions were described by Rodriguez-Leor et al., in whose study mechanical thrombectomy was performed significantly more often in STEMI patients with confirmed SARS-COV2 virus infection compared to controls (44% vs. 33.5%, p = 0.046) [22]. In our analysis, STEMI and COVID+ patients were more likely to be both in a serious condition and to experience out-of-hospital sudden cardiac arrest. The study group of patients undergoing aspiration thrombectomy required the use of more intensive anticoagulant therapy. During PCI they were more often subjected to thrombolysis; moreover, a satisfactory effect (TIMI 3) was less often obtained than in the control group. In this work, among the predisposing factors for aspiration thrombectomy in STEMI patients enrolled in the study were COVID-19, Killip-Kimball class IV, male gender, and smoking.
The prothrombotic state described in the course of COVID-19 infection and the difference in its course compared to coagulation disorders previously known may worsen the course of ACS and require a different therapeutic approach. In the early stages of the pandemic, according to some authors, fibrinolysis may have been a reasonable alternative to STEMI treatment [23]. The authors of a large review concluded that intracoronary thrombolytic drugs significantly improved myocardial perfusion and significantly reduced the occurrence of serious adverse cardiovascular events without increasing the risk of bleeding compared to aspiration thrombectomy [24]. Polish research on the phenomenon of thrombectomy also brings important data. Siudak et al. in the NRDES study, which involved 13 hemodynamic labs, divided patients into two large groups: those who were treated with thrombectomy at the time of primary PCI and those not treated with this method. The study involved 2686 patients. As a result, thrombectomy in patients undergoing primary PCI in STEMI was not associated with an improvement in long-term, 1-year clinical outcome [25].
The results regarding the time from first medical contact to balloon inflation or angiogram in patients with STEMI and COVID-19 in the available literature are contradictory. Although most studies indicate that this time was longer compared to the controls [26, 27], some authors did not find significant differences in this respect [28]. In our analysis, the time from first contact to balloon inflation or angiogram was significantly longer for STEMI patients with co-infection with COVID-19 compared to controls, but the median ischemia time was similar in both groups. The reasons for this state of affairs could be e.g. the patient’s fears of internal infection, operator covers, lack of barrier clothing available at the beginning of the pandemic, or longer time of patient transport to the interventional cardiology center. In the regression analysis, time from first contact to balloon inflation or angiogram had a significant but small effect on the occurrence of thrombectomy.
Summing up these reports, it seems that the hyperinflammatory response generated by COVID-19 infection strongly affects changes in the coagulation system, which can cause hypercoagulability and related complications. The fact that in patients with STEMI and COVID-19 non-obstructive coronary artery disease is more common, aspiration thrombectomy is more frequently performed, incidence of stent thrombosis is higher, and higher doses of anticoagulants are necessary raises the question of what the actual role of the coagulation and fibrinolytic systems is in the context of this disease and how this knowledge can be applied for potential risk-based benefits. It seems that in the case of patients with STEMI and COVID-19 who did not show a coronary vascular lesion of the nature of concomitant atherosclerotic plaque during PCI, thrombectomy, intravascular thrombolysis, without stent implantation, may be a promising direction of treatment, especially since stent thrombosis was more common among patients diagnosed with COVID-19. In the group with concomitant atherosclerotic lesions, the best solution seems to be the use of thinner stents, covered with a polymer with the lowest possible thrombogenicity. In addition, it remains to be determined which therapy affecting the coagulation system would be the most optimal, taking into account the fact that despite more intensive anticoagulant treatment in the group of COVID-19 STEMI patients (+), PCI treatments were less likely to result in an optimal clinical outcome (TIMI 3).
Summing up these data and current knowledge, in the course of COVID-19 and STEMI infection, in some cases a different pathophysiology of coronary vascular lesion formation should be taken into account, and the occurring coagulation disorders and shifting the balance in the procoagulation direction can be treated as a consequence of the inflammatory reaction generated in the course of SARS-CoV2 coronavirus infection. This may lead to a different therapeutic approach to this group of patients. Unlike many other studies on thrombectomy in STEMI with concomitant COVID-19, our study considered perioperative mortality (instead of in-hospital mortality), which was similar in both groups. The study presented here is the first to focus directly on examining the factors affecting eligibility for thrombectomy. When analyzing the factors influencing the eligibility for thrombectomy, we considered STEMI with a short time from the onset of pain to FMC and from FMC to angiography (< 24 h). COVID-19 independently increased the risk of thrombectomy.
Study limitations
No techniques were used to adjust for baseline differences in patients of the 2 unequal groups such as propensity matching. Whether a patient was included in the STEMI COVID-19 (+) group was determined by the positive result of the antigen test performed in the ambulance or at the destination hospital. Swabs for molecular RT-PCR were always performed before the procedure; however, due to the urgency of the intervention, its results were not waited for. There is no database that presents these data, which is a limitation of this study.
Conclusions
COVID-19 (+) STEMI patients undergoing aspiration thrombectomy were more often in a severe clinical condition (higher Killip-Kimball class, more often pre-procedure cardiac arrest) than COVID-19 (–) patients. Despite more intensive antiplatelet and antithrombotic treatment, PCI procedures less often resulted in an optimal TIMI 3 effect. COVID-19 is an independent strong predictor of patients’ qualification for aspiration thrombectomy in STEMI.








