Introduction
Background
Burn survivors may experience ongoing dissatisfaction even after the primary burn injury has healed, as scarring often persists. Hypertrophic burn scarring, characterized by elevated scars in the burn area, can profoundly impact a survivor’s body image and functional abilities. Predicting who will develop scarring is challenging. Research indicates that less severe burns, healing in under 14 days, typically result in no scarring, whereas more serious burns may take 14 to 21 days to heal and may leave scars. Burns necessitating skin grafting and requiring over 21 days for healing are highly likely to result in scarring. Factors such as the location, depth, and age of the burn all influence the extent of scarring. When the dermal or lower layer of the skin is damaged, scarring occurs as the body produces collagen to repair the skin. However, hypertrophic scars exhibit disorganized collagen fibres, altering the texture and appearance of the newly formed skin or scar. Normally, collagen fibres are deposited in a relatively orderly manner. Scar healing typically requires time. Scarring typically begins within a few months, peaks at 6 months, and resolves or “matures” within 12 to 18 months [1]. During the reconstruction phase of wound healing, the replacement of normal tissue with fibrous tissue inevitably leads to the formation of scars. Initially, collagen is produced in a random manner, resulting in bulky fibres that later undergo remodelling along the lines of strain. This process may lead to adhesions in nearby tissues. Over time, these collagen strands are replaced by stronger, better-organised collagen, resulting in a flatter, smoother scar with a lighter coloration [2]. An estimated 180,000 fatalities worldwide are attributed to burns annually, with approximately two-thirds of these cases occurring in the African and Southeast Asian regions as designated by the World Health Organization (WHO). In low- and middle-income countries, the number of burn-related child fatalities has surged by sevenfold compared to high-income countries, despite declining burn fatality rates in many affluent nations. Non-fatal burns represent a significant cause of morbidity, leading to prolonged hospitalizations, disabilities, psychological distress, and societal stigmatization. Moreover, burns rank among the leading causes of diminished disability-adjusted life years (DALYs) in low- and middle-income nations [3]. In India, there are over a million reported cases of moderate-to-severe burn injuries annually. Similarly, approximately 173,000 Bangladeshi youngsters suffer from mild severe burns each year. Among children with burn injuries in Egypt, Pakistan, Bangladesh, and Colombia, 17% experience temporary disabilities, while 18% endure permanent ones. Burns rank as the second most common type of injury in rural Nepal, comprising 5% of all injuries. In the United States, more than 410,000 burn injuries were reported in 2008, with approximately 40,000 requiring hospitalization [4].
Significance and challenges in scar management
Burns inflict skin damage that leads to the formation of burn scars. With burns that affect only the surface layers of the skin, scar tissue gradually diminishes over time. However, damage to the deeper layers of the skin results in more enduring scarring, which may appear thick, leathery, or uneven. Severe burn scars often manifest as raised, red, purple, or pink scars, referred to as hypertrophic scars. Hypertrophic scars (HS) commonly occur following burns, surgeries, cuts, and other traumatic injuries.
Keloids and hypertrophic scars represent varying manifestations of excessive dermal fibrosis, believed to stem from dysregulated cell proliferation and turnover during wound healing in predisposed individuals. These entities differ in clinical presentation, histological characteristics, and cellular responses to molecular stimuli. Both conditions result from heightened fibroblast activity and increased deposition of extracellular matrix components. The interaction between epidermal keratinocytes and dermal fibroblasts is pivotal in modulating tissue equilibrium and the formation of scars. Keloids and hypertrophic scars represent distinct phases of a shared process, each characterized by unique clinical and histological features.
Deep second-degree burn wounds penetrate deeply into the dermis and take a long time to cure, leading to 50%–83% frequency of hypochromia. Scars not only cause pain, itching, malfunction, and other discomfort to varied degrees in patients, but they can also negatively impact their mental and physical well-being [5], especially in youngsters as they are still growing and developing. The management and prevention of HS in burn victims remain challenging. Similarly, addressing hypovolemic shock in burn patients poses ongoing difficulties. Traditional approaches encounter obstacles when patients decline participation anti-scar treatment, and pressure therapy necessitates consistent pressure application throughout specific treatment stages, limiting its therapeutic efficacy. Conventional scar treatment methods face limitations in effectively compressing scars in joint areas as many patients are hesitant to actively engage in anti-scar therapy. Despite numerous advancements in medical science offering various scar reduction options, the removal of atrophic burn scars poses particular challenges, making their treatment exceptionally challenging.
Scar tissue make-up
The majority of scars consist of fibrotic tissue, distinct from healthy skin, which gives them their unique texture and appearance. This altered composition makes restoring the natural contours of the affected area more challenging. Fibrotic tissue lacks the collagen and elastin fibres typically found in healthy skin, further complicating the restoration process.
The level and range of scarring
The severity of burn injuries can significantly influence the extent and severity of resulting scarring. Deeper burns that penetrate the skin’s dermis or subcutaneous layers often lead to more severe scarring. Achieving significant scar reduction in larger affected areas is more challenging due to the increased complexity of integrating new tissue and regenerating healthy skin cells.
Contracture
One of the common side effects of burn scars is scar contracture, where the surrounding skin tightens and compresses as scar tissue forms. This can lead to limitations in movement and functional impairments, particularly if the scars are located near joints or critical body areas. Effective treatment of scar contracture requires a comprehensive approach addressing both the surface appearance of the scar and the tightness of the underlying tissue.
Inadequate source of blood
Damage to blood vessels during the burning process often leads to compromised blood supply in severe burn scars. This diminished blood flow hampers the delivery of vital nutrients and oxygen necessary for proper healing and regeneration in the injured area. Consequently, the restricted blood supply makes the excision of atrophic burn scars more challenging and contributes to delays in the healing process.
Specific factors
In the treatment of severe burn scars, the outcomes are influenced by individual circumstances. Factors such as age, overall health, and underlying medical conditions can affect the body’s ability to heal. Additionally, the skin type and pigmentation may impact the effectiveness of certain therapies. Therefore, personalized treatment programs tailored to each patient’s unique needs are crucial for achieving the best outcomes.
Aim
Material and methods
Literature review
Scarring can impose constraints on both appearance and function. Effective scar treatment necessitates carefully planned regimens incorporating specialised therapeutic techniques. Implementing a conceptual framework for scar treatment enhances patient safety and streamlines clinical practice for practitioners. While numerous methods and approaches exist for scar care, the emergence, development, and consequences of scar tissue for both function and appearance remain significant concerns in the healthcare industry. With the global scar treatment market projected to exceed $32 billion by 2027, scar treatment remains a prominent issue across the medical sector [6]. Scar treatment might include simple conservative measures or more complex procedures that need to be individually tailored to each patient. Improving functional and aesthetic abnormalities is the primary goal of scar therapy [7, 8]. Currently, there is no universally accepted, evidence-based treatment protocol for addressing aesthetically and functionally problematic scars. The intricate pathophysiology of scar formation, absence of suitable model systems for evaluating treatment efficacy, difficulties in quantifying changes in scar appearance, and limited availability of prospective, randomised controlled trials underscore the need for further research in scar therapy. Consequently, clinical practice has largely relied on individual experience rather than standardized professional consensus. In 2014, Gold et al. proposed the most recent method for scar treatment [9]. Here, conventional therapy algorithms were mostly discussed without including sophisticated surgical scar treatment possibilities.
Scar management
Conservative methods of therapy
The best ways to get flat, pliable, and aesthetically pleasing scars are through conservative treatment methods, including scar massaging and pressure therapy using silicone-containing scar plasters or compression dressings [10]. Moreover, PT and OT are essential therapeutic pillars for functional rehabilitation [11]. Scar tissue typically undergoes spontaneous improvement as it ages. Scar revision procedures are often performed 6 to 12 months after the scar initially forms. However, in complex and long-term cases, conservative therapy approaches may fail to yield satisfactory outcomes and may not be sustainable, necessitating surgical intervention. Following comprehensive medical education regarding available treatments and associated risks, patients and physicians collaborate to determine the need and timing for surgical procedures. The selection of an appropriate surgical technique remains highly individualized, contingent upon various factors such as the scar’s location, skin texture, size, and colour. It is crucial to ensure that acute tissue inflammation has resolved before proceeding with surgery.
Therapeutic lasers
Laser therapy is one effective and safe first treatment for traumatic scars and contractures. The development of pathological scars and the accompanying impairment may be reduced by early laser therapy [12]. CO2 lasers, Er:YAG lasers and ablative lasers are commonly utilized to treat hypertrophic scars [13]. This course of treatment aims to break down collagen and encourage the formation of new collagen. However, ablative laser therapy may result in pigment abnormalities, keloid development, persistent erythema, oedema, and extended downtime [14]. Fewer side effects are associated with non-ablative lasers than ablative lasers, which also induce collagen remodelling. Because of the higher level of extracellular remodelling, ablative lasers often produce better results than non-ablative lasers. Ablative lasers have more adverse effects than non-ablative lasers, which additionally trigger collagen remodelling. Generally speaking, ablative lasers produce better results than non-ablative lasers because of the higher level of extracellular remodelling.
Injectable triamcinolone
Keloids and hypertrophic scars provide unique challenges for scar therapy. Injections of trimethylene acetate have proven to be short-term effective. One of the most popular forms of therapy is still their intra-lesional administration. They are probably effective because they cause fibroblast hypo-activity, reducing collagen production and decreasing fibroblast density. In addition, triamcinolone seems to inhibit blood vessel endothelial bud development [15]. Nevertheless, new research indicates that up to 50% of keloids may not respond to triamcinolone infusions and may exhibit a considerable recurrence after the first response [16, 17].
Fat-filling
Lipo-filling techniques are included in the autologous fat transplantation for scars. Patients with painful, hypertrophic, or retracting scars should especially consider these. The procedure, based on autologous fat extraction, is often performed under general anaesthesia. Following fat processing, the leftover fatty tissue is injected behind the scar in the hypodermis. Overall, lipo-filling operations are minimally invasive treatments that help ease the strain in scar tissue. Additionally, a scar’s aesthetic look can be enhanced [18].
Medicinal needles
Medical needling works based on percutaneous collagen induction, whereby pressure is applied to the target region using needle rollers, creating a series of micro-wounds in the dermal layer. Under local or general anaesthesia, the process initiates a posttraumatic inflammatory cascade while protecting the epidermal structures, facilitating collagen production and skin regeneration [19]. It has developed into a dependable technique for bigger scar tissue regions and, in contrast to the laser and externally ablative treatments, is safe and efficient for rejuvenation operations and wrinkle treatment. It also does not cause harm to the epidermis. As a result, repeated medical needling treatments might produce the best outcomes.
Skin grafting
Tissue repair can be effectively and attractively achieved using skin transplants. Important choices in skin grafting are whether to use split or full-thickness grafts, as well as what texture and pigmentation match works best for the recipient site [20]. Despite the fact that full-thickness grafts are particularly helpful for repairing face defects after scar excision, split-thickness grafts are advised for fixing big defects and in places where skin contraction is relatively favourable for lowering the size of the defect [21]. Mesh skin grafts also have the potential benefit of expanding the skin graft, lowering donor-site morbidity, and preventing fluid retention in recipient locations that are sick [22].
DRT templates: dermal regeneration
When tissue abnormalities cannot be adequately repaired with split-thickness skin grafts, dermal templates are utilized. In addition to providing a physical barrier to stop wound infections, they act as a scaffold, encouraging tissue regeneration and prompt wound closure. Benefits include the skin’s ability to regain its flexibility and suppleness while maintaining an appropriate level of durability [23].
Flaps
Local flaps: Z-plasty and other local flap treatments can be used to reposition scar tissue and place it deeper into loosened skin tension lines. One advantage is that the scar becomes less noticeable due to the deregulation caused by the scar tissue breaking apart. With Z-plasty, a variety of tension vectors are offered to assist prevent scar hypertrophy and contraction [24].
Vascularized flaps: Regional flaps, such as myocutaneous or vascularize pedicle fascio-cutaneous flaps, are employed when scar removal creates a gap that is visible by adjacent tissue. Regional flaps are fraught with complications, including the potential donor-site morbidity, and are recommended only in cases of severe functional or aesthetic impairment.
Free tissue transfer flaps: Free tissue transplantation has emerged as one of the most effective treatment modalities for large wounds, thanks to advancements in medical technology and microsurgical operating techniques. New options that enable a wide range of tissue to be transplanted to even the most remote regions of the human body have been developed as a result of improved understanding of free flap plasty, including flap form, blood flow, and flap delay. For free tissue transfer, any tissue – including skin, fat, muscle, fascia, bone, and tendon – can be used [25].
Fractional CO2 laser in scar treatment
Doctors and academics generally embrace the new scar therapy procedure that has changed all these. In contrast to conventional non-surgical techniques, laser technology offers advantages, including short treatment times, less trauma, speedy recovery, ease of use, and good effectiveness [12, 26]. Laser technology has been applied in medicine since the 1980s. Several different lasers are now available for scar therapy, including fractional CO2 lasers and pulsed dye lasers [27]. Various lasers have varying degrees of scar-reduction efficiency. The use of CO2 fractional laser in wound healing, preventing overgrowth of scars, and reducing discomfort and itching associated with scars has been the subject of several research in last few years [28]. Fractional CO2 laser has demonstrated efficacy in treating HS and reducing scar-related pruritus, as demonstrated by Huang et al. [29]. Miletta et al. [30] have shown that a fractional CO2 laser operating at a wavelength of 10600 nm has demonstrated varying degrees of success in treating scars, as supported by the hypothesis of photo-thermal breakdown. The redness, swelling, and hardness of scars can be significantly improved by fractional CO2 laser, according to Tan et al. [31] treatments of 221 patients with post-burn HS. Furthermore, the advent of fractional CO2 lasers has made it possible to do less ablative resurfacing, which rectifies abnormalities in the scar surface and its pliability while reorienting dermal collagen in all skin types. These CO2 lasers filter out particles, creating micro-fractionated laser beams. This is in contrast to non-ablative lasers, which only cause ablation of the epidermis in particular tissue areas. Forty-eight hours later, the injured regions spontaneously heal, and the ensuing tissue remodelling causes the collagen to reorganize, the scars to flatten, and the surface to become uneven [32].
The mechanism of action involves two primary processes: thermal wounding and collagen remodelling [33].
Thermal wounding: The CO2 laser emits light at a specific wavelength of 10,600 nanometres. This light is readily absorbed by water in the skin cells, leading to the precise removal of the superficial skin layer. This process is also known as ablation [34].
Collagen remodelling: The thermal wound induced by the CO2 laser triggers a healing response in the skin. This response stimulates the production of new collagen, a protein that provides structure and elasticity to the skin [34]. The new collagen helps to smooth out the skin’s surface and reduce the appearance of scars and wrinkles [34].
It is important to note that while CO2 laser therapy is highly effective, it is associated with certain risks and side effects [35]. Therefore, proper patient selection, pre-, peri-, and postoperative instructions, and management of complications are crucial [35].
Hirudoid guided by ultrasound
Scars on the face, keloids (a type of scar that appears after an injury has healed), hypertrophic scars (red scars caused by an excess of collagen at the injury site), and other scar types can all be treated using a topical medication called Hirudoid cream. In order to reduce pain and relax tight muscles, therapeutic ultrasound uses low-power ultrasonic waves to create vibrations and raise the temperature of body tissue in a particular area. The technique can help break up scar tissue and enhance blood and lymph circulation to aid in the healing of the injured region. It is usually applied to minor orthopaedic injuries and persistent pain [36]. Two main methods of applying ultrasound to skin exist: either the skin is pretreated with ultrasound before coming into touch with a medication or permeant, or the drug or permeant is applied via a coupling medium.
Hirudoid gel, a topical treatment used for various skin conditions, works through several mechanisms. It acts as a local anti-coagulant, preventing blood clotting and reducing inflammation to promote healing. The main ingredient, mucopolysaccharides (MPS), is similar to the naturally occurring MPS in the human body’s dermis, facilitating the skin’s healing process. The gel promotes the regeneration of skin tissues, speeding up the healing process and reducing swelling and inflammation [37]. It also improves blood flow to the affected area, delivering nutrients and oxygen to the skin cells. Furthermore, Hirudoid gel increases the production of collagen and elastin fibres in the connective tissue, improving the skin’s appearance and texture. It enhances the skin’s moisturizing capacity, keeping the skin hydrated and promoting healing. The gel also increases the content of hyaluronic acid, a substance that helps retain moisture in the skin, contributing to skin hydration, volume, and elasticity. Lastly, it accelerates the absorption of bruises, reducing their appearance faster.
In contrast, the skin is simultaneously treated with ultrasound. The augmentation of drug transport that results from simultaneous treatment is caused by two processes: an increase in skin permeability due to structural changes in the skin and convection-related mechanisms specific to ultrasound [38]. In comparison, the pretreatment technique only increases skin permeability through a mechanism as the medication is administered after the completion of ultrasound therapy. The benefits of ultrasonography therapy for burn scars are increased blood flow, improved collagen tissue extensibility, and raised pain thresholds. When topical therapeutic ultrasound is applied to scar tissue, it has been observed that this raises the tissue temperature, increasing collagen’s extensibility. By administering ultrasound to the targeted region and/or the transducer head of the ultrasound in combination with Hirudoid cream or gel (a topical anti-inflammatory drug). They employ circular movements to glide the transducer over the desired skin area. Depending on how deep the injured tissue is located and how well the injury is mending, the ultrasonic machine’s settings can alter the intensity or penetration depth during therapy.
Methodology
This section comprises the process and methods involved during this study to effectively address the notion of using fractional CO2 laser with Hirudoid in burn scar management.
Study design
The research design provides the pattern and structure of the study, which it will follow during the conduct. In this conduct, the design employed was quasi-experimental. A quasi-experimental design was chosen because it allows for comparing different groups under controlled conditions, even if random assignment is not possible in hospital settings.
Participants
The study comprised 20 participants including 16 female and 4 male participants. To more accurately assess the outcomes, the patients were divided into two groups: a control group and an experimental group. Moreover, participants age range was 13–50. For this study, participants were selected from Ruian People’s Hospital, Ruian 325200, Zhejiang, China and were all post-burn scar patients. The experimental group was treated with fractional CO2 laser therapy after applying Hirudoid under ultrasound guidance. Whereas, the control group was treated with regular conventional scar management strategies.
Inclusion criteria
The age range of 13 to 50 years
Burn scars of at least 20 cm2 and at least a year old
Ready to take part in the research
Individuals who have scars from burn surgery
Exclusion criteria
History of individuals with hypertrophic scars or keloids
Participants who had any kind of medical intervention in the previous 6 months, particularly a systemic retinoid
Prior history of laser therapy-related side effects or a condition that precludes fractional CO2 laser treatment
Women who are nursing or pregnant
Individuals whose burn scars are limited to their heads and necks
Patients with actively infected scar area
Data collection
The study recruited burn scar patients seeking treatment at East Jeddah General Hospital between March 2022 and August 2023. A total of twenty patients were enrolled, comprising 16 women and 4 men, aged between 13 and 50 years, with an average age (insert mean age ± standard deviation [SD]). Information regarding the source, location, length, and prior treatment methods of each subject’s burn scars was recorded.
The experimental group underwent three treatment sessions involving a fractionally ablative 10,600 nm CO2 laser and therapeutic ultrasound-guided Hirudoid applied to target scars. These sessions were spaced 4 to 8 weeks apart. Before the laser procedure, the target region was treated with a topical anaesthetic for 30 to 60 min, followed by thorough cleaning and drying. During the laser treatment, the following settings were applied in a single pass: stacking = 3, power = 18 W, dwell duration = 600 s, and spacing = 200 m. Post-laser home therapy protocol included the application of topical panthenol 2% twice a day for 4 weeks, with instructions to avoid crust removal and to apply sunscreen frequently to scars located in sun-exposed areas.
In contrast, the control group received conventional scar management procedures. Throughout the study period, clinical assessments, including evaluation of scar characteristics, pain levels, and patient-reported outcomes, were conducted at regular intervals.
Primary outcome measures
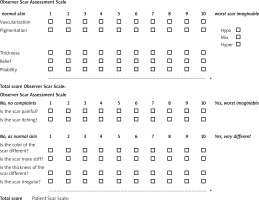
Measures of the primary outcome were the Vancouver Scar Scale (VSS) (Table 1) [35] and Patient and Observer Scar Assessment Scale (POSAS) (Figure 1) [36]. Both were computed 2 months following the final laser treatment and at baseline. The VSS score and the observer’s share of the POSAS was computed by the lead investigator. The patient’s section of the POSAS was completed by each participant. Additionally, ratings for the patient’s overall assessment were computed on a scale from 1 to 10. Next, the sum of the POSAS scores was determined.
Table 1
Vancouver Scar Scale (VSS) [36]
Secondary outcomes measures
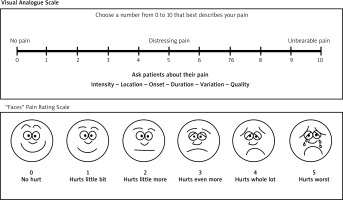
However, patient satisfaction, pain levels throughout therapy, functional outcomes, and unfavourable consequences of the therapies were included in the secondary outcome measures. The visual analogue scale (Figure 2) [39, 40] was used to evaluate patient satisfaction, and pain levels. Moreover, the passive and active range of motion exercises were also performed to assess functional outcomes, including scar flexibility and the range of motion.
The scoring of the patient’s improvement has been done using a rating scale of five, such as:
shows the patient did not improve,
shows the patient improved satisfactorily,
denotes the patient improved good,
shows the patient improved better,
shows the patient improved excellently.
These scoring criteria have been chosen to better and accurately identify the patient’s improvement from the given treatment and comprehend the effectiveness of the fractional CO2 laser with Hirudoid in comparison to conventional scar management.
Statistical analysis
The collected data were analysed using SPSS software version 27, employing statistical methods and tests to ensure a thorough and effective examination in line with the study’s objectives. Descriptive statistics, including standard deviations, means, variance, and minimum and maximum values, were utilized to gain insight into the average improvement of each group. This facilitated the summarization and comparison of overall participant improvements under different conditions, while also allowing for an examination of score distribution and confirmation of data meeting the assumptions of parametric tests.
To compare test scores between the experimental and control groups, the study employed the t-test, which is appropriate for comparing means across different time points and treatment groups. This statistical test assists in determining whether disparities in improvement between patient groups are statistically significant. The significance threshold was set at p < 0.05, indicating a significant deviation from normal distribution.
Results
In 11 (55%) patients, thermal burns caused by hot fluids constituted the source of burn scars, while a total of 9 (45%) patients experienced burns from other sources. Among the patients, 14 (70%) had scars on their arms, 10 (50%) on their lower extremities, 2 (10%) individuals on their chest, 1 (5%) patient on their breast, and 1 (5%) patient on their abdomen. The duration of scars ranged from 1 to 30 years, with a mean duration of 12.30 ±8.70 years. Prior therapeutic techniques included intra-lesional steroids, grafting, surgical release, and topical therapy (Table 2).
Table 2
Clinical data of the patients
Experimental group
Table 3 displays the scores obtained by the experimental group following assessment using the Vancouver Scar Scale (VSS) subsequent to fractional CO2 laser treatment with Hirudoid. The assessment encompasses vascularity, pigmentation, pliability, and height. The experimental group comprised 10 participants, with 3 participants demonstrating good improvement (score of 3), 5 participants showing much better improvement, and 2 patients exhibiting excellent improvement.
Table 3
Vancouver Scar Scale (VSS) scores of the experimental group
| Score | Frequency | Percent | Valid percent | Cumulative percent | |
|---|---|---|---|---|---|
| Valid | 3 | 3 | 27.3 | 27.3 | 27.3 |
| 4 | 5 | 45.5 | 45.5 | 72.7 | |
| 5 | 2 | 18.2 | 18.2 | 90.9 | |
| VSS Score | 1 | 9.1 | 9.1 | 100.0 | |
| Total | 11 | 100.0 | 100.0 | ||
Table 4 presents the scores obtained by the experimental group following assessment using the Patient and Observer Scar Assessment Scale (POSAS) post-treatment. The assessment included both an observer’s section, comprising vascularity, pigmentation, thickness, relief, and pliability, and a patient’s section, which involved questions regarding scar pain and perception. Within the experimental group consisting of 10 patients, 6 patients demonstrated better improvement (score of 4), while 4 patients exhibited excellent improvement.
Table 4
Patient and Observer Scar Assessment Scale scores of the experimental group
| Score | Frequency | Percent | Valid percent | Cumulative percent | |
|---|---|---|---|---|---|
| Valid | 4 | 6 | 54.5 | 54.5 | 54.5 |
| 5 | 4 | 36.4 | 36.4 | 90.9 | |
| POSAS | 1 | 9.1 | 9.1 | 100.0 | |
| Total | 11 | 100.0 | 100.0 | ||
Table 5 illustrates the scores derived from the Visual Analogue Scale (VAS) assessment conducted post-scar treatment within the experimental group. The assessment involved a pain rating scale ranging from 0 to 10, indicating varying degrees of pain from none to unbearable. Within the experimental group comprising 10 individuals, 3 patients demonstrated better improvement (score of 4), while 7 patients exhibited excellent improvement.
Control group
Table 6 indicates the frequency of the control group, in which the scores gained by the control group is indicated by this table. In addition, as from the table, 7 and 3 patients improved the assessment on a scale of 2 and 3, respectively. These scales indicate that 2 denotes patient improved satisfactorily, 3 shows participants improved good.
Table 6
Vancouver Scar Scale (VSS) for the control group
| Score | Frequency | Percent | Valid percent | Cumulative percent | |
|---|---|---|---|---|---|
| Valid | 2 | 7 | 63.6 | 63.6 | 63.6 |
| 3 | 3 | 27.3 | 27.3 | 90.9 | |
| VSS Score | 1 | 9.1 | 9.1 | 100.0 | |
| Total | 11 | 100.0 | 100.0 | ||
The frequency of the control group is shown in Table 7, which also shows the scores that the control group obtained. Furthermore, according to the table, 9 patients and 1 patient improved their assessment on a scale of 2 and 3, respectively. Based on these measures, a patient’s improvement was deemed satisfactory if their score was 2, and good if it was 3.
Table 7
Patient and Observer Scar Assessment Scale (POSAS) for the control group
| Score | Frequency | Percent | Valid percent | Cumulative percent | |
|---|---|---|---|---|---|
| Valid | 2 | 9 | 81.8 | 81.8 | 81.8 |
| 3 | 1 | 9.1 | 9.1 | 90.9 | |
| POSAS | 1 | 9.1 | 9.1 | 100.0 | |
| Total | 11 | 100.0 | 100.0 | ||
The results of the Visual Analogue Scale (VAS) evaluation for the control group are displayed in Table 8. Ten people made up the control group; of these, 7 patients showed satisfactory improvement and 3 showed good improvement.
Table 8
Visual Analogue Scale (VAS) for the control group
| VAS | Frequency | Percent | Valid percent | Cumulative percent | |
|---|---|---|---|---|---|
| Valid | 2 | 7 | 63.6 | 63.6 | 63.6 |
| 3 | 3 | 27.3 | 27.3 | 90.9 | |
| VAS | 1 | 9.1 | 9.1 | 100.0 | |
| Total | 11 | 100.0 | 100.0 | ||
The descriptive statistics provided in Table 9 offer additional information regarding the data for the experimental and control groups in this research, which evaluates the effects of fractional CO2 laser with Hirudoid in burn scar management.
For the experimental group, the range values indicate scores ranging from a minimum of 3 to a maximum of 5, suggesting substantial variation in improvement among patients within the group. The mean scores for each assessment were 3.90, 4.40, and 4.70, respectively, indicating that, on average, individuals in the experimental group scored above the midpoint of the 1 to 5 scale, signifying improvement between “better” and “excellent”. Moreover, the relatively low standard deviations of 0.737, 0.516, and 0.483, respectively, suggest a low degree of variability in scores within the experimental group, indicating a consistent treatment effect across participants. Similarly, the low variances of 0.544, 0.267, and 0.233, respectively, indicate consistent improvements within the experimental group.
Table 9
Descriptive statistics of both groups
In contrast, for the control group, the range values show scores ranging from 2 to 3, with mean scores of 2.3, 2.1, and 2.3, respectively, slightly below the scale’s midpoint. On average, patients in the control group performed slightly below the level of “good”. The standard deviations of 0.483, 0.316, and 0.483, respectively, indicate less variability in scores within this group, with variances of 0.233, 0.100, and 0.233, respectively, corresponding to the low standard deviations.
In summary, the experimental group performed well above the scale’s midpoint, with low variability in scores, suggesting a positive impact of the fractional CO2 laser combined with ultrasound-guided Hirudoid intervention. In contrast, the control group performed slightly below the scale’s midpoint, with relatively consistent scores, indicating a lesser impact of the intervention. These results suggest that the intervention had a more significant and positive impact on the performance of the experimental group compared to the control group.
The fractional CO2 laser intervention was linked to ultrasound-guided Hirudoid surgery in the experimental group (n = 10). In contrast, the control group (n = 10) had a history of traditional scar care. An independent sample t-test was conducted to examine the therapeutic effectiveness of fractional CO2 laser in conjunction with ultrasound-guided Hirudoid. As Table 10 illustrates, both groups’ distributions were sufficiently normal to support the t-test. Levene’s F-test was also used to test and confirm the assumption of homogeneity of variances, yielding F(20) = 0.55, 12.054, and 0.000 for each assessment scale, respectively. A statistically significant impact was found to be related to the independent sample t-test t(20) = 5.737, 12.01, and 11.11 of each, respectively, p < 0.001). Hence, compared to the control group, the experimental group was linked to a statistically significant increase.
Table 10
Independent sample t-test for both groups
Discussion
By using fractional CO2 laser therapy with ultrasound-guided Hirudoid, objective measurements demonstrated a major improvement in the burn scars as well as a significant shift in the patients’ perceptions of the scars’ appearance [41]. The results obtained by many researchers using various settings were consistent. Relief and pliability showed much greater improvement in the present research, with vascularity and pigmentation following closely behind. This conclusion was consistent with that of Kim et al. [42], who found that the use of an ablative fractional CO2 laser was superior in improving the thickness and pliability of surgical scars. On the other hand, vascularity and pigmentation responded well to treatment with a pulsed dye laser (PDL). This suggests that the best candidates for fractional CO2 laser therapy are scars that are solid and uneven rather than erythematous and hyper-pigmented. Treatment for hyperaemic scars may be more successful if fractional CO2 laser is used initially, followed by PDL targeting the vasculature. The use of fractional CO2 in our study resulted in a significant improvement in scar thickness, pliability, and comfort, as evidenced by the administration of Hirudoid by therapeutic ultrasonography.
Based on our research, fractional CO2 lasers produce superior results when the scar is shorter in duration. Scars that are younger than a year old show more obvious recovery. The primary cause of this is the effect of cytokines and growth factors on fibroblast activity during the initial phases of wound healing. In contrast, studies have demonstrated that the patient’s age or the location of their scar had no bearing on the percentage of clinical recovery. The length, age, or anatomical placement of the scar did not have an impact on the effectiveness of the therapy [43]. It was typically bearable in terms of side effects and problems. Hyperpigmentation improved with Hirudoid, same as in our conditions. Skin regeneration may lead to a notable lightening of scars, as shown in the case of 1 patient in this study. Only in 2 cases actual hypopigmentation occur. After the patient prematurely removed the crust, the second case experienced hypopigmentation. Comparing the current study to other earlier research, the incidence of hypopigmentation is significantly lower. The substantial decline in the overall evaluation ratings of patients’ POSAS suggests that, in general, the side effects that emerged did not impact patients’ satisfaction with the outcomes that were obtained. To sum up, fractional CO2 laser combined with Hirudoid has shown to be a safe and effective therapy for scars left behind after burns. Patients’ judgments of how their scars seem are considerably changed by it.
Limitations: This research has a few limitations, which are listed as follows:
The sample size used in this study may not fully mirror the country’s entire population, potentially limiting the generalizability of the findings.
The research’s outcomes may not be broadly applicable due to the study’s narrow scope and the specific group of participants involved.
This study concentrated on Jeddah hospital patients, which could constrain the transferability of the results to other contexts or patient populations.
Additional sessions are required to get the best answer.
It was not possible to minimize adverse effects and prevent confluent thermal damage because of narrow separation.
Future research
Future studies in the field of scar management in burn patients can be taken into consideration. Future scholars can investigate the long-term consequences of using fractional CO2 laser in combination with Hirudoid.
They can also undertake comparative research across various hospitals and countries to evaluate the generalizability of the findings of fractional CO2 laser and Hirudoid impact on burn scar management.
Including other traditional therapy elements – the clinical effectiveness of fractional CO2 laser and Hirudoid in treating scars caused by burns – can provide valuable information.
Future researchers can consider and develop additional sessions of treatment to achieve the required best answer of the notion.
In addition, they should focus using a wider separation to minimize adverse effects and prevent confluent thermal damage.
Conclusions
Scarring is a common and often distressing consequence of burn injuries, posing significant challenges to both patients and healthcare providers. This study aims to assess the clinical efficacy of a novel therapeutic approach, combining ultrasound-guided Hirudoid application with fractional CO2 laser treatment, in the management of scars resulting from burn repair procedures. The research involves a comprehensive investigation into the impact of this combined therapy on scar appearance, patient satisfaction, and functional outcomes. The use of Hirudoid, a topical medication containing heparinoid, aims to mitigate inflammation and improve tissue healing, while fractional CO2 laser therapy is employed for its ability to promote collagen remodelling and reduce scar hypertrophy. Preliminary results indicate a significant improvement in scar texture, pigmentation, and overall appearance in the experimental group compared to the control group. Patient-reported outcomes demonstrate enhanced satisfaction with the combined therapy, and functional assessments suggest potential benefits in terms of scar flexibility and the range of motion. Additionally, adverse effects and safety profiles of the combined therapy are thoroughly evaluated to ensure its feasibility and tolerability in a clinical setting.
The study contributes valuable insights into the development of more effective and patient-friendly interventions for scar management post-burn repair. In conclusion, the investigation into ultrasound-guided Hirudoid combined with fractional CO2 laser therapy represents a promising avenue for improving the clinical outcomes of burn scar treatment. The integration of advanced technologies and therapeutic modalities may pave the way for enhanced scar management strategies, ultimately improving the quality of life for individuals affected by burn injuries. It significantly alters the patients’ perceptions of how their scars look. Our study’s shortcomings were a limited sample size and a very brief follow-up time. To get the best answer, many sessions are required. To lessen side effects and prevent confluent thermal damage, a wider spacing might be employed. The occurrence of hyperpigmentation can also be decreased by using bleaching treatments concurrently.