Summary
The MitraClip system has been shown to provide low peri-procedural complication rates, a significant reduction in mitral regurgitation (MR) with mortality benefits, improved functional capacity, and improved quality of life in high-surgical-risk patients with degenerative (primary) or functional (secondary) MR. However, data on the change in mitral valve annular diameter (MAD), left atrial appendage (LAA) structure, and function after transcatheter edge-to-edge repair (TEER) of the mitral valve in patients with secondary MR are lacking. We aimed to evaluate the change in these parameters after the MitraClip procedure, and its relationship with prognosis in the long term, and found that the contraction and filling velocity of LAA, systolic pulmonary artery pressure, MAD, and LAA landing zone dimension before and after the procedure are useful parameters that can be used to evaluate the effectiveness of the MitraClip procedure. Although these parameters are not related to composite outcome in our study, more comprehensive and longer studies are needed.
Introduction
MitraClip (Abbott Laboratories, Menlo Park, California, USA) is a system developed for the transcatheter edge-to-edge repair of the mitral valve in patients with severe mitral regurgitation (MR) and high-risk for surgery [1]. In previous studies, the MitraClip system has been shown to provide low peri-procedural complication rates, a significant reduction in MR with mortality benefits, improved functional capacity, and improved quality of life in high-surgical-risk patients with degenerative (primary) or functional (secondary) MR [2–4]. MitraClip is a catheter-based technology similar to the surgical Alfieri technique used in MR treatment [1]. The first clinical use of MitraClip in humans was carried out in 2003 and the number of cases worldwide has approached almost 200,000 [1, 5]. MitraClip is performed via the femoral vein access, consisting of a steerable catheter and a clip delivery system under the guidance of 3D TEE.
The EVEREST II trial was ground-breaking as the first randomized trial to compare transcatheter edge-to-edge mitral valve repair (TEER) with surgical repair/replacement in patients with degenerative and functional MR [2]. This study compared surgical repair with TEER at 12 months for mortality and severe MR as the primary composite endpoint, demonstrating not only that the MitraClip procedure was a safe treatment option, but also that secondary MR as well as primary MR was effectively treated with MitraClip [2]. Subsequently, the MITRA-FR and COAPT studies investigated the clinical efficacy and safety of TEER in addition to guideline-directed optimal medical treatment in patients with heart failure and severe secondary MR [3, 4]. While the MITRA-FR study [3] found similar rates of all-cause death and unplanned hospitalization for heart failure between the Mitra-Clip plus optimal medical therapy (OMT) and OMT-only groups, the COAPT study [4] showed promising results for the MitraClip plus OMT group.
Mitral valve morphology, and clinical and anatomical eligibility criteria should be carefully assessed with TEE for a successful MitraClip procedure. Many parameters such as a decrease in the MR grade, spontaneous echo contrast formation, mitral valve gradient and left atrial pressure, changes in pulmonary vein flow velocities, and increased stroke volume at the end of the procedure have been used to evaluate the success and prognosis in the short term [6–9]. However, additional practical parameters that can provide information about short- and long-term success are needed.
Aim
In our study, we aimed to investigate the changes in anterior-posterior mitral valve annulus diameter (MAD), left atrial volume, and flow characteristics as procedural success indicators after the MitraClip procedure, and the relationship between these parameters and prognosis.
Material and methods
Patient selection
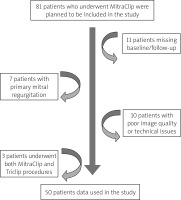
This study was approved by the Yeni Yuzyil University Institutional Review Board (IRB #2022/07-889), and written informed consent was obtained from all subjects participating in the trial. Mitral valve repair was performed with the MitraClip system in the patients included in the study from June 2019 to June 2021. The study included patients with severe secondary or mixed type MR (MR ≥ 3+), who are at high surgical risk (STS score ≥ 8), are symptomatic despite optimal medical therapy, and meet the anatomical and clinical eligibility criteria for MitraClip. A cardiac team comprising a cardiologist specializing in mitral valve interventions, a cardiovascular surgeon specializing in valve surgery, and an anaesthesiologist evaluated the patients. Patients with active endocarditis, rheumatic valve disease, mean mitral valve gradient above 5 mm Hg, and intracardiac/inferior vena cava/femoral vein thrombus were excluded from the study. Patients with comorbid diseases that would prevent the expected benefit from postprocedural MR reduction were also excluded. Initially, 81 patients were included in the study; however, it was designed with the data of 50 patients for the reasons stated in the flow chart (Figure 1).
Echocardiographic assessment
Echocardiographic evaluation of the patients was performed following the current guidelines [10, 11]. Echocardiographic studies were performed by 2D and 3D data with a commercially available echocardiographic system (Philips CX50 Ultrasound Machine, Amsterdam, Netherlands) and echocardiography probes (S5-1 for TTE, X7-2t x-Matrix for TEE). The evaluation of the degree of MR with TEE was determined by the regurgitant volume, regurgitation fraction, effective regurgitant orifice area (EROA), and Doppler vena contracta. LAA measurements were performed using biplane windows with 35–55° and 125–145° views. The landing zone was defined as the LAA internal diameter one centimetre away from the circumflex artery. Two measurements were performed in each view, and the maximum diameter was included in the analysis. LAA flow was interrogated by pulsed-wave Doppler. The sample volume was placed within the proximal third of the appendage, located within the LAA cavity, to avoid wall motion artifacts. Filter and gain settings were adjusted to obtain optimal LAA flow recordings from which LAA contraction and filling velocities were measured. At least 3 measurements in sinus rhythm and 5 measurements in atrial fibrillation rhythm were obtained. The mean of the measurements was recorded as filling and contraction velocity. MAD was measured in mid-diastole and long-axis view (anterior-posterior). 3D echocardiographic imaging was used during clip placement (Figure 2).
Percutaneous repair procedure
TEER was performed using the MitraClip device in the cardiac catheterization laboratory in all patients. The procedure was performed under general anaesthesia with fluoroscopic and TEE guidance. Atrial transseptal puncture was performed in a posterior and mid-to-superior location on the interatrial septum after confirmation of adequate distance from puncture location to the mitral annular plane. After atrial transseptal puncture, the device was steered until aligned with the origin of the regurgitation jet and advanced into the left ventricle. Adequate reduction of mitral regurgitation to 2+ degrees or less on echocardiography was considered a successful procedure. If the reduction in MR was insufficient with a single device, a second clip was inserted. Unfractionated heparin was administered for the systemic anticoagulation during the procedure, and the activated clotting time was monitored to keep it above 300 s.
Statistical analysis
The distribution of the parameters was assessed using the Shapiro-Wilk test. Continuous variables were expressed as mean and SD or median and interquartile range based on the distribution of the parameters. Categorical variables were expressed as frequency and percentage. Two-group comparisons were performed using the χ2 test or Fisher exact test for categorical variables: Student’s t test or Mann-Whitney U test were used for continuous variables. Where appropriate, measurements at 2 time points were compared with paired Student’s t-test or Wilcoxon signed-rank test. Spearman correlation analysis was used to compare left atrium (LA) and LAA measurements at baseline and follow-up. A multivariate Cox regression was performed to evaluate the independent significance of all parameters with a p-value of < 0.05 in univariate analysis. Statistical analysis was performed using SPSS software version 25.0 (SPSS, Inc, Chicago, IL). A p-value of 0.05 was considered statistically significant.
Results
A total of 50 patients were analysed in our study, including 35 (70%) males and 15 (30%) females, with a mean age of 71.5 years. The procedural success of the patients included in the study was 100%. The mean follow-up period of the patients was 10.5 ±8.9 (1–36) months, and no in-hospital mortality was observed. Demographic data of the patients are presented in Table I. The functional capacity of all patients varied with New York Heart Association (NYHA) III or IV. The number of patients using sacubitril-valsartan and sodium-glucose co-transporter-2 (SGLT-2) inhibitors was 17 (34%) and 23 (46%), respectively. Eighty percent of the patients had ischaemic heart failure, and 38% had an implantable cardioverter-defibrillator (ICD) or cardiac resynchronization therapy (CRT) device. The mean STS score was 16.8 ±3.5%, and the EurosSCORE II was 18.2 ±4.1%.
Table I
Baseline clinical characteristics of the study population (n = 50)
[i] ACE – angiotensin-converting enzyme, AF – atrial fibrillation, ARB – angiotensin II receptor blocker, BMI – body mass index, BNP – brain natriuretic peptide, CAD – coronary arterial disease, CRF – chronic renal failure, CRT – cardiac resynchronization therapy, DM – diabetes mellitus, GFR – glomerular filtration rate, HT – hypertension, ICD – implantable cardioverter defibrillator, MRA – mi-neralocorticoid receptor antagonist, NYHA – New York Heart Association, SGLT-2 – sodium-glucose cotransporter-2, STS – Society of Thoracic Surgeons.
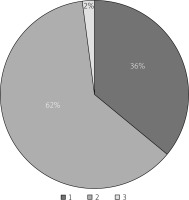
Baseline TTE and pre- and post-MitraClip TEE data are presented in Table II. The aetiology of MR was functional in 84% and mixed type in 16% of patients. The mean left ventricular ejection fraction was 25.9 ±6.6%. MR was grade 4 in 78% of patients and grade 3 in 22%. A single clip, 2 clips, or 3 clips were applied in 18 (36%), 31 (62%), and one (2%) patient/s, respectively (Figure 3). A mean of 1.66 ±0.6 clips implanted per patient (range: 1 to 3).
Table II
Baseline echocardiographic characteristics of the study population (n = 50)
[i] EF – ejection fraction, EROA – effective regurgitant orifice area, LAD – left atrial dimension, LAVI – left atrial volume index, LVEDD – left ventricular end-diastolic dimension, LVEDVI – left ventricular end-diastolic volume index, LVESD – left ventricular end-systolic dimension, MR – mitral regurgitation, PASP – pulmonary artery systolic pressure, RF – regurgitation fraction.
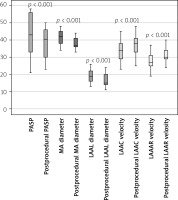
TEE imaging of the patients revealed that LAA contraction and filling velocity significantly increased after MitraClip (pre-clip: 34.2 ±5.9 cm/s, 28.2 ±4.3 cm/s; after clip: 37.3 ±6.0 cm/s, 31.0 ±4.5 cm/s (p < 0.001 for both)). SPAP, MAD, and LAA landing zone dimension significantly decreased (respectively, pre-clip: 43.6 ±11.4 mm Hg, 4.1 ±0.44 cm, 19.0 ±3.2 mm; post-clip: 38.1 ±9.6 mm Hg, 3.9 ±0.36 cm, 16.2 ±3.4 mm (p < 0.001 for all)). There was no significant change in mitral valve maximum and mean pressure gradients before and after MitraClip (pre-clip: 6.3 ±2.0 mm Hg, 3.1 ±0.9 mm Hg; post-clip: 6.4 ±1.4, 3.2 ±0.7, (p = 0.67, p = 0.46)). Results in terms of primary outcomes are shown in Table III, and data showing significant change after MitraClip are shown in Figure 4 in the box plot graph.
Table III
Overall changes of echocardiographic indices post-operatively
| Parameter | Pre-operative | Post-operative | Correlation coefficient | P-value |
|---|---|---|---|---|
| Mitral annular diameter [cm] (mean ± SD) | 3.9 ±0.38 | 3.6 ±0.46 | 0.92 | < 0.001* |
| LAA landing zone dimension [mm] (mean ± SD) | 19.0 ±3.2 | 16.2 ±3.4 | 0.84 | < 0.001* |
| LAA contraction (outflow) wave [cm/s] (mean ± SD) | 34.2 ±5.9 | 37.3 ±6.0 | 0.85 | < 0.001* |
| LAA filling (inflow) wave [cm/s] (mean ± SD) | 28.2 ±4.3 | 31.0 ±4.5 | 0.84 | < 0.001* |
| PASP [mm Hg] (mean ± SD) | 43.6 ±11.4 | 38.1 ±9.6 | 0.92 | < 0.001* |
| Mean pressure gradient [mm Hg] (mean ± SD) | 3.1 ±0.9 | 3.2 ±0.7 | 0.54 | 0.46 |
| Peak pressure gradient [mm Hg] (mean ± SD) | 6.3 ±2.0 | 6.4 ±1.4 | 0.76 | 0.67 |
Figure 4
The box plot graph compares pulmonary artery systolic pressure, mitral annular diameter, LAA landing zone, and LAA velocities before and after MitraClip
LAAC – left atrial appendage contraction, LAAF – left atrial appendage filling, LAAL – left atrial appendage landing, MA – mitral annular, PASP – pulmonary artery systolic pressure.
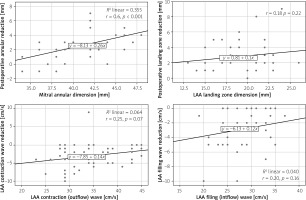
The multivariate Cox regression analyses showed that there was only a significant correlation between the MAD before clip placement and the MAD change after clip placement (r = 0.6, p < 0.001) (Figure 5).
Figure 5
Scatter graph of Spearman correlation analysis results showing the relationship between pre-procedure values of mitral annular diameter LAA landing zone and LAA flow velocities and post-procedure measurement changes
LAA – left atrial appendage.
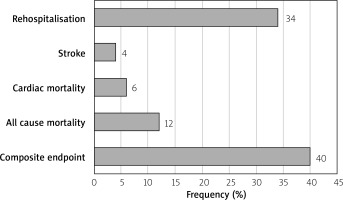
During a mean follow-up period of 10.5 ±8.9 months, one or more hospitalizations for heart failure occurred in 17 (34%) of the patients, stroke occurred in 2 (4%) patients, and cardiac mortality occurred in 3 (6%) patients. While all-cause mortality occurred in 6 (12%) patients, the composite endpoint consisting of rehospitalisation and all-cause mortality occurred in 23 (46%) patients (Figure 6). No significant correlation was found between MAD change, LAA contraction and filling velocity change, LAA landing zone dimension change and rehospitalization, stroke, mortality, and composite outcome.
Discussion
The significant results of our study are as follows: i) LAA contraction and filling velocity significantly increased after MitraClip; ii) SPAP, MAD, and LAA landing zone dimension significantly decreased after MitraClip; and iii) there was no relationship between MAD and LAA size and velocity change with rehospitalization, stroke, mortality, and composite outcome in the long term.
Several studies have found that MR is associated with poor outcomes regardless of aetiology [12–14]. This poor prognostic effect starts from mild levels of MR and increases with its severity [14]. Following current guidelines, medical treatment is the first and indispensable treatment step in MR. To date, 3 randomized studies have investigated the efficacy and safety of MitraClip in patients with advanced MR. The EVEREST II study’s 5-year follow-up results revealed no significant difference in mortality between the MitraClip and surgery groups. (20.8%, 26.8%, respectively). Thus, at long-term follow-up, the results improved in favour of MitraClip compared to surgery [15]. The MITRA-FR study’s 2-year follow-up data revealed that, in addition to OMT, MitraClip did not produce a significant reduction in hospitalization rate or mortality when compared to OMT alone [16]. According to the COAPT study’s 3-year follow-up results, MitraClip therapy in patients with low EF and secondary MR, who are symptomatic despite OMT, provides a permanent reduction in MR compared to OMT alone, reduces hospitalizations due to heart failure, improves quality of life and functional capacity, and reduces mortality [17].
There are several factors that could explain the difference between the early and long-term results of the MITRA-FR and COAPT studies. The number of patients, selection criteria, study design, and technical elements varied between these 2 studies. First of all, the patients enrolled in the COAPT study had more severe MR (EROA 41 ±15 mm2 vs. 21 ±10 mm2) and less left ventricular dilatation (mean left ventricular diastolic index 101 ±34 ml/m2 vs. 135 ml/m2) compared to MITRA-FR patients [3, 4]. In terms of EF, the inclusion criteria of patients in the MITRA-FR and COAPT studies were quite different (15–40% vs. 20–50%) suggesting that the MITRA-FR study included patients with more advanced stage of LV dysfunction. In many studies, patients with ischaemic MR of severe LV dilatation (LV end-diastolic diameter > 65 mm) and LV dysfunction (LVEF < 20%, LV systolic diameter > 55 mm) have shown a higher rate of persistent or recurrent MR, less reversal LV remodelling, and worse outcomes after surgery [18, 19]. In our study, there was no difference in composite outcome in the long term after the procedure, as the patients mostly followed the MITRA-FR study in terms of the EF value as the inclusion criterion.
Many studies have been conducted to evaluate the role of new parameters related to early and long-term success [8, 20–22]. The post-procedure residual MR is the most crucial of these. In sub-analyses of the COAPT study, residual MR was evaluated 30 days after the procedure. Patients with ≤ 2 grade residual MR were found to have less hospitalization, better quality of life, and less mortality at 2-year follow-up than those with 3+/4+ residual MR [8]. Mitral valve gradient after the procedure is another parameter that has been investigated. In COAPT trial [20], the patients were divided into three groups according to the final mean mitral valve gradients. The analysis results showed no difference between the groups regarding adverse events over the 2-year follow-up. The findings also pointed out that the benefit of reduced MR overcame the effect of mild-to-moderate mitral stenosis.
Kuwata et al. [21] analysed continuous left heart pressure recordings during the procedure. In this analysis, post-procedure mean left atrial (LA) pressure and left ventricular end-diastolic pressure decreased, and left ventricular end-systolic pressure increased significantly. More than moderate residual MR and an increase in the LA mean pressure index after the procedure were associated with poor prognosis in long-term follow-up [21]. Because measurement of the left atrial pressure by catheterization is impractical, we proposed that new parameters be used. To the best of our knowledge, this is the first study investigating the relationship between the combination of MAD, SPAP, LAA descent zone diameter, and left atrial flow velocities with procedural success and long-term prognosis.
The pulmonary vein flow pattern has also been used to assess the severity of MR and LA pressure [22]. Ikenega et al. [22] assessed the procedure based on the pulmonary flow pattern. A rapid increase in systolic pulmonary velocity following the successful MitraClip procedure was revealed. According to this analysis, the ratio of systolic to diastolic pulmonary velocity could be utilized to evaluate the procedural success.
Our study has several limitations. First of all, the study was retrospective and single centre. Second, the relatively small number of patients and the short follow-up period make it difficult to evaluate statistically in this respect. Third, because the study included patients with low ejection fraction, the effect of MitraClip on patients with higher ejection fraction could not be evaluated.
Conclusions
The MitraClip procedure offers an improved functional capacity and quality of life with low peri-procedural complication rates and a significant reduction in MR severity. The contraction and filling velocity of LAA, SPAP, MAD, and LAA landing zone dimension before and after the procedure are useful parameters that may be used to evaluate the effectiveness of the MitraClip procedure. Because the TEER procedure was performed with 100% success in the study, the results of the study cannot be used to predict the effect of successful and unsuccessful procedures on MAD and LAA diameters and velocities. The results show the effect of the TEER procedure only in terms of the acute effect on the MAD and LAA diameters and velocities.