Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease, which affects both children and adults. Atopic dermatitis is associated with pruritus, lichenification, chronically relapsing course and atopic history, personal or familial [1–3]. The origin of AD is complex; genetic, immunologic and environmental factors may be involved[3, 4]. Its development and progression are highly connected with a skin barrier dysfunction, resulting in increased transepidermal water loss, higher propensity to microbial infections or facilitation of allergen skin penetration [5, 6]. The course of dermatitis remarkably affects the quality of life of patients and their families[7, 8].
Treatment of AD is complex and includes daily skin care, allergen avoidance or use of topical remedies [3, 5]. Currently, topical corticosteroids (TCS) are the first-line therapy for patients with AD[9]. Their efficacy is briefly proven; nonetheless, AD is a disease with a chronic course and usually requires long-term, constant treatment. Long-standing TCS therapy presents a clear risk of adverse events (AEs) [4, 9]. Alternative treatment options with fewer side effects are being considered to overcome this issue, revealing calcineurin inhibitors (TCI) as a brilliant replacement for topical treatment. However, comparing with TCS, they are novel remedies in AD treatment and despite indisputable efficacy, the risk of AEs is still in question. Nowadays they are used as a second-line therapy option[10, 11].
Aim
This review aimed at determination if TCI are a superior alternative for TCS and comparison of these two therapies in terms of their efficacy and safety. The study is of great importance to help establish novel guidance to AD therapy, treatment of the disease affecting an increasing number of people.
Material and methods
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA)[12]. No review protocol was registered.
Search strategy and data collection
A systematic review of the Cochrane Central Register of Controlled Trials (CENTRAL) (of 1980), MEDLINE via Ovid (of 1946), EMBASE via Ovid (of 1988), EMBASE via Ovid (of 1988), Global Resource of Eczema Trials (GREAT database) databases was performed up to 22 February 2018. Search terms included (eczema OR neurodermatitis OR atopic dermatitis) AND (tacrolimus OR protopic OR fk506 OR pimecrolimus OR elidel). Six trials registers were searched (meta Register of Controlled Trials, US National Institutes of Health Ongoing Trials Register, Australian New Zealand Clinical Trials Registry, World Health Organization International Clinical Trials Registry platform, EU Clinical Trials Register, Ongoing Skin Trials Register). Bibliographies of identified articles were manually screened to find further references to relevant studies.
Study selection
Searched records were merged to remove duplicates, followed by examination of titles and abstracts of the remaining trials. Selected relevant studies were fully read for compliance with the eligibility criteria. Inclusion criteria were as follows: (1) randomized controlled trials, (2) people diagnosed with AD by a physician or other specialist using standardized diagnostic criteria of Hanifin and Rajka[1], (3) comparison of TCI and TCS treatments, (4) inclusion of at least one outcome of interest.
Pre-specified primary outcomes included physician’s global assessment of improvement and occurrence of AEs. Pre-specified secondary outcomes included efficacy of treatment assessed by a validated or objective measure: affected Body Surface Area (BSA), Eczema Area and Severity Index (EASI) and modified EASI (mEASI). Studies which did not provide any data concerning efficacy or safety were excluded from analysis. Subgroup analysis was performed for mid-strength to potent and least potent to lower mid-strength TCS. For missing or unavailable data, sponsors websites or clinical trials reports were searched to derive needed information. Whenever possible, results from intention-to-treat (ITT) population were used.
Quality of assessment
Quality of evidence provided by the studies was evaluated using Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria [13]. The risk of bias analysis was performed using the Cochrane Collaboration Risk-of-Bias Tool for randomized controlled trials[14]. It incorporated the following aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of the outcome assessment, analysis of incomplete outcome data, selective reporting, and other biases.
Statistical analysis
The risk ratio (RR) and 95% confidence intervals (CIs) were calculated for dichotomous outcomes. For continuous variables with a similar scale, mean differences (MD) and 95% CIs were calculated. If outcomes for continuous data used different scales, standardized mean differences (SMD) and 95% CIs were calculated. When the study did not provide necessary information, it was omitted in a part of analysis. Results were considered to be statistically significant if 95% CIs did not include the null value (RR = 1) and p≤ 0.05. Heterogeneity across studies was assessed using I2 statistics [14], which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error. If subgroup or total I2 was assessed > 50%, indicating possible substantial heterogeneity, a random effects model was used, otherwise the fixed model was applied. Analysis of data was conducted using Review Manager 5.3 (The Cochrane Collaboration).
Results
Study selection
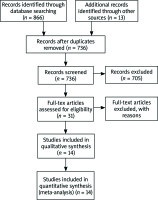
Electronic search, shown in Figure 1, revealed 736 non-duplicative individual studies, from which 705 did not meet inclusion criteria after abstract screening. The remaining 31 were examined as a full text, excluding other 17 articles. Finally, 14 studies were incorporated in qualitative and quantitative analysis.
Characteristics of the studies
Table 1 summarizes characteristics of included articles. All included studies were randomized controlled trials. A total number of 7376 participants were included into analysis. Corticosteroids therapy comprises various potency drugs, eight studies [15–22] examined least potent to lower mid-strength corticosteroids and only five [2, 23–26] mid-strength to potent drugs. Twelve studies [2, 15–23, 26] addressed both primary outcomes, namely physician’s global assessment of improvement and AEs, one [25] addressed AEs only. One study described secondary outcomes only [27].
Table 1
Characteristics of included trials
| Study | Therapy | N | Duration [weeks] | Location | Age of participants |
|---|---|---|---|---|---|
| Bieber 2007 [26] | Tacrolimus 0.03% | 136 | 3 | Multi-centre | Children |
| Methylprednisolone aceponate 0.1% | 129 | ||||
| Doss 2009 [2] | Tacrolimus 0.1% | 288 | 3 | Multi-centre | Adults |
| Fluticasone 0.005% | 280 | ||||
| Doss 2010 [24] | Tacrolimus 0.03% | 240 | 6 | Multi-centre | Children |
| Fluticasone 0.005% | 239 | ||||
| Hofman 2006 [18] | Tacrolimus 0.03% | 121 | 28 | Multi-centre | Children |
| Hydrocortisone acetate 0.1% and hydrocortisone butyrate 1% | 111 | ||||
| Luger 2001 [25] | Pimecrolimus 1% | 45 | 3 | Multi-centre | Adults |
| Betamethasone valerate 0.1% | 42 | ||||
| Luger 2004 [23] | Pimecrolimus 1% | 328 | 52 | Multi-centre | Adults |
| Triamcinolone acetonide 0.1% and hydrocortisone acetate 1% | 330 | ||||
| Mandelin 2010 [22] | Tacrolimus 0.1% | 40 | 52 | Single-centre | Adults |
| Hydrocortisone butyrate 0.1% and hydrocortisone acetate 1% | 40 | ||||
| Neumann 2008 [27] | Tacrolimus 0.1% | 20 | 87 | Single-centre | Adults |
| corticosteroids regimen | 20 | ||||
| Reitamo 2002a [26] | Tacrolimus 0.03% or Tacrolimus 0.1% | 189/186 | 3 | Multi-centre | Adults |
| Hydrocortisone acetate 1% | 185 | ||||
| Reitamo 2002b [15] | Tacrolimus 0.03% or Tacrolimus 0.1% | 193/191 | 3 | Multi-centre | Children |
| Hydrocortisone butyrate 0.1% | 186 | ||||
| Reitamo 2004 [17] | Tacrolimus 0.03% | 210 | 3 | Multi-centre | Children |
| Hydrocortisone acetate 1% | 207 | ||||
| Reitamo 2005 [19] | Tacrolimus 0.1% | 487 | 26 | Multi-centre | Adults |
| Hydrocortisone butyrate 0.1% and hydrocortisone acetate 1% | 485 | ||||
| Sigurgeirsson 2015 [20] | Pimecrolimus 1% | 1205 | 260 | Multi-centre | Children |
| Hydrocortisone acetate 1% and hydrocortisone butyrate 0.1% | 1213 | ||||
| Sikder 2005 [21] | Tacrolimus 0.03% | 15 | 4 | Multi-centre | Children |
| Clobetasone butyrate 0.05% | 15 |
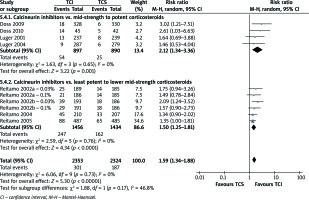
Physician’s global assessment of improvement: clear or excellent (Figure 2)
Neither comparison of mid-strength to potent TCS with TCI nor least potent to lower mid-strength TCS showed significant results (RR = 1.03, 95% CI: 0.95–1.12; RR = 1.39, 95% CI: 0.90–2.16). Otherwise, collective analysis of these two comparisons indicated that TCI therapy is slightly more effective than TCS one (RR = 1.24, 95% CI: 1.06–1.44). In order to support described results, quantitative analysis of secondary outcomes, EASI, mEASI and affected BSA, was planned. EASI is an instrument used to score the extent and severity of AD. Its compositions cover ratings of four signs: erythema, oedema/induration/papulation, excoriations, lichenification affected BSA[28]. mEASI is a modification of EASI, which additionally includes assessment of itch[19, 22]. BSA simply describes the percentage of the area affected by AD. Unfortunately, data reported in included studies were incoherently presented or some of them lacked details such as standard deviations, standard errors, mean difference or confidence interval. Consequently, quantitative analysis was impossible to carry out. However, few trials reported significant differences between examined treatments. All these trials examined least potent to lower mid-strength corticosteroids. TCI treatment was reported to cause a greater improvement in terms of mEASI in two studies [16, 19] (p< 0.01), in terms of BSA in four studies [15, 18, 19, 21] (p< 0.05) and EASI in two studies [19, 22] (p< 0.05). Albeit, TCS treatment was shown to cause a better mEASI improvement in one study [21] (p = 0.018), BSA improvement in another study [20] (p≤ 0.006) and EASI improvement in also another one [23] (p≤ 0.006).
Figure 2
Physician’s assessment of global response of improvement: clear or excellent. For citation references, see Table 1
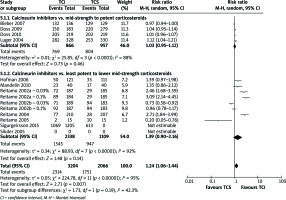
Overall number of AEs
The outcomes were addressed in all studies comparing mid-strength to potent TCS and four studies comparing least potent to lower mid-strength TCS. Any of these comparisons or pooled estimate of them did not produce significant results (Figure 3).
Skin burning and pruritus events
A number of studies [2, 15, 17, 19, 21, 24] indicated skin burning and pruritus as the most common AEs accompanied with AD treatment. TCI therapy in all computed comparisons (Figures 4 and 5) cause more skin burning or pruritus events (RR = 3.32, 95% CI: 2.90–3.80; RR = 1.59, 95% CI: 1.34–1.80, respectively).
Discussion
Long-standing research of AD reveals many novel options for its treatment with TCI as an example. The efficacy of TCI treatment is undisputed [4], albeit their safety were called into question in 2005, when the US FDA recommended a ‘black box’ warning, which represents serious or life-threating risks. The indication was improperly assigned because of the insufficient data concerning long-time safety and risk of cancer [29]. Currently, TCI are recommended as a second-line therapy [30], while they should be considered on an equal level as the alternative option for TCS. Consequently, studies examining TCI safety on a larger population of patients are expedient. This study aimed at efficacy and safety examination of TCI therapy in comparison with standard corticosteroids therapy. The review included only data comparing TCI with TCS, leaving behind similar comparisons for example of tacrolimus vs. a combination of TCS and tacrolimus[21]. The current review showed a slight dominance of TCI over TCS in terms of efficacy (Figure 2) when comparing TCI with both defined subgroups: least potent to lower mid-strength or mid-strength to potent TCS. Unfortunately, TCI failed to demonstrate greater safety, its treatment elicits a higher number of AEs (Figures 4 and 5). Additionally, primary outcomes were not supported by additional evidence (EASI, mEASI or affected BSA). Results presented in the current study are in accordance with the ones published earlier [4, 31–37]. The adjudication equivalence might have happened because the majority of included trials were common for all meta-analysis. Nevertheless, until now this study has included the largest number of children and adults raising the advantage over the former studies. Some of meta-analyses mentioned above focused only on paediatric patients [31, 34, 36], placed pimecrolimus as a control group [4, 31, 34], lacked data concerning safety [32] or efficacy of treatment [36]. One review [35] focused on pro-reactive treatment despite the reactive one. Many of them included the vehicle into comparison [31–33], while that evaluation does not express the decision making process between choosing TCI or TCS.
The current review examined 7376 patients with moderate or severe AD, all participants applied topical ointments twice daily. Group sizes were various and ranged from 15 to 1213 participants, although sizes of pooled populations were close (3894 patients applying TCI, 3482 corticosteroids). The methodological quality of 14 trials, based on risk of bias assessment, was good. All studies were free of other sources of bias and did not report their outcomes selectively. Eleven out of 14 trials were investigator-blinded ones, in 12 blinding of participants or personnel were described. Only two studies did not mention any operation to deal with incomplete outcome data. Random sequence generation was not described in one trial. Allocation concealment was not reported in majority of trials. Quality of evidence questions the results of current review. Main outcomes evaluating the efficacy were assessed to provide very low quality of evidence assessed using GRADE score. Adverse events (skin burning or pruritus) outcomes were estimated to have moderate quality. These results were probably induced by different characteristics of trials with an example of diversified age among participants in examined studies. Both adults (at least 16 years old) [2, 16, 19, 22, 23, 25, 27] and children (2–15 years) [15, 17, 18, 20, 21, 24, 26] were incorporated. Surprisingly, despite age-dependent treatment recommendations, no substantial differences between children and adults were observed in this review. Only one study [17] incorporating children and two incorporating adults [16, 23] revealed TCI treatment to be significantly more effective than TCS only. Safety analysis, presented in Figures 3–5, demonstrated more consistent results. Another limitation of this analysis, which might induce the quality of results, were different follow-up times applied in included studies: short-term (a few weeks) [2, 15–17, 21, 24–26] and long-term [18–20, 22, 23, 27] observations were assessed together. More serious AEs, with skin atrophy as a convenient example[30], appears after chronic use, what cannot be assessed in short-term observations. Skin atrophy is a result of steroid-dependent suppression of collagen synthesis in connective tissue. Calcineurin inhibitors are shown to have greater specificity and no impact on connective tissue [38]. Despite weaknesses presented above, the power of the current review might be considered substantial. Reviews were carried out on a population of 7376 and revealed statistically significant differences in treatment efficacy in favour of TCI and in treatment safety in favour of TCS.
Conclusions
TCI treatment might be slightly more efficient than AD treatment. Contrarily they are associated with more incidences of AEs, such as skin burning or pruritus. Albeit, standardized recommendations for reporting outcomes and interventions should be developed to ease the analysis of a subject in question. Another issue, which impedes the analysis, is still too small number of long-term trials. Along with a greater number of existing trials, more variables, like age of participants, follow-up time or drug potency, could be accommodated into meta-analysis. Complex analysis, incorporating these variables simultaneously, would provide credible safety and efficacy data, and consequently novel guidance for AD therapy.