Introduction
Significant stenosis of the left main (LM) coronary artery is one of the most challenging subsets for percutaneous coronary intervention (PCI). This fact is related to high anatomical complexity, along with a large myocardial ischemic territory, which increases the risk of periprocedural complications and affects the long-term results [1]. Nevertheless, despite the initial high Syntax Score, features such as advanced age and comorbidities commonly result in an unacceptably high surgical risk of coronary artery bypass grafting (CABG), leaving PCI as the last available revascularization option.
Calcifications are among the strongest predictors of the unfavorable short- and long-term outcomes of PCI. The severity of coronary calcification affects procedure safety and efficacy mostly by an increased probability of suboptimal stent expansion, which in turn is inseparably linked with a higher rate of target lesion failure, mainly in-stent restenosis and stent thrombosis.
Historically, calcium modification was achieved by a high-pressure dilatation of non-compliant balloons or cutting/scoring balloons. However, both methods in terms of heavy calcifications show limited effectiveness. Recently, a novel debulking device – the Diamondback 360° Coronary Orbital Atherectomy (OA) System (Cardiovascular Systems Inc., Saint Paul, USA) – has demonstrated relatively good safety and efficiency [2, 3]. However, data regarding the LM disease are scarce [4, 5]. This paper evaluates the initial experience with the Orbital Atherectomy System used as a debulking device in high-risk patients with calcified LM disease.
Case reports
Case 1
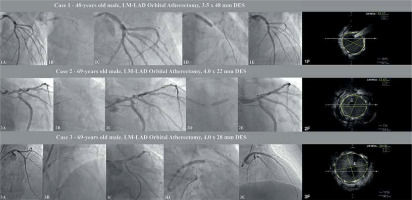
A 48-year-old man with hypertension, hyperlipidemia, heart failure with reduced left ventricular ejection fraction (HFrEF) with left ventricle ejection fraction (LVEF) ~25% and a history of coronary artery disease (CAD), i.e. past PCI-RCA with drug-eluting stent (DES) implantation, was admitted to hospital due to non-ST elevation myocardial infarction (NSTEMI). Coronary angiography revealed significant stenosis of the LM and the proximal part of the left anterior descending (LAD) artety with a co-existing high calcium burden. Due to lack of consent for the proposed surgical treatment, this patient underwent rescue PCI. The procedure was performed via right radial access and the EBU 3.5 6 F guiding catheter was used. After advancing the Viper Wire (Cardiovascular Systems Inc., Saint Paul, USA) to the distal part of the LAD the 1.25 mm diamond-coated crown of the orbital atherectomy device was advanced to the proximal part of the LAD with Glide Assist mode. We performed eight atherectomy runs lasting 25 s, conducted backward (from the distal to the proximal part of the lesion), at low speed (80 000 rpm), and additionally four runs for 25 s at high speed (120 000 rpm). Afterwards, we performed additional, successful high-pressure (18 atm) 3.5 × 15 non-compliant NC balloon lesion preparation, which was followed by DES implantation (3.5 × 48 mm) under IVUS guidance, at 14 atm. Subsequently, the proximal optimization technique (POT) with a 5.0 × 8 mm (20 atm) NC balloon was performed and the final angiogram with additional intravascular ultrasound (IVUS) confirmed adequate stent apposition.
Case 2
A 69-year-old man with persistent atrial fibrillation, COPD, diabetes, HFrEF (LVEF – 25–30%) with coexisting CAD treated in the past with PCI of the right coronary artery (RCA) was admitted to the hospital due to stable angina (CCS II). Coronary angiography revealed significant stenosis of the LM and proximal LAD with a high calcium burden. The patient was referred to the local heart team, and due to a high comorbidity rate with a coexisting low ejection fraction of the left ventricle was disqualified from CABG. Therefore, PCI was performed via right radial access (6F EBU 3.5). After wiring the LAD with Viper Wire, the OA crown with the support of Glide Assist mode (5000 rpm) was placed in the medium part of the LAD. Initially, two low-speed (80,000 rpm) backward runs (from medium LAD to LM) were performed. Afterwards, in a similar direction, six high-speed (120,000 rpm) runs with subsequent post-dilation with a 4.0 mm NC balloon (18 atm) were conducted. In the next step, under IVUS guidance DES (4.0 × 22 mm) an additional POT (5.0 × 8 mm, 19 atm) was performed with a reasonable angiographic result confirmed in IVUS.
Case 3
A 69-year-old obese (BMI 39 kg/m2) man with hypertension, diabetes, hyperlipidemia, and CAD with a history of multiple PCI (LAD, Cx and RCA) and heart failure with preserved ejection fraction (HFpEF) was admitted to the hospital due to unstable angina. Initial angiography revealed critical stenosis of the RCA (restenosis) and significant, calcified LM disease. Hence, we performed drug-eluting balloon (DEB) angioplasty of the RCA with a 3.0 × 20 mm paclitaxel DEB. Two days later the patient underwent PCI of the LM via 6F right radial access. After wiring the LAD, we performed 6 (25 s) successful, low-speed (80,000) anterograde runs (from the LM into the proximal LAD). In the next part of the procedure, high-pressure (18 atm) inflation with a 3.5 × 15 mm NC balloon with subsequent implantation of the 4.0 × 28 mm DES was performed. After the IVUS assessment, we used additional POT using a 5.5 × 8mm NC balloon (Figure 1).
Figure 1
Selected procedures of OA-LM-PCI: 1A – initial LM lesion, 1B – orbital atherectomy (OA) burr passage, 1C – post-OA control angiogram, 1D – DES implantation, 1E – final post-PCI angiogram, 1F – final IVUS assessment, 2A – initial LM lesion, 2B – orbital atherectomy (OA) burr passage, 2C – post-OA control angiogram, 2D – DES implantation, 2E – final post-PCI angiogram, 2F – final IVUS assessment, 3A – initial LM lesion, 3B – orbital atherectomy (OA) burr passage, 3C – post-OA control angiogram, 3D – DES implantation, 3E – final post-PCI angiogram, 3F – final IVUS assessment
Discussion
Rotational atherectomy (RA), since its introduction to clinical practice, has been proven to be more successful than standard balloon predilation in calcified lesions [6] and has become a gold standard in the aggressive lesion preparation. Despite the relatively high effectiveness, several shortcomings of this method may be noted [7]. RA is a rather demanding procedure with a “long” learning curve requiring a high level of training and technical expertise. During the RA various complications, including coronary artery dissection, perforation, vasospasm, slow-flow/no-reflow, or burr entrapment, may occur.
Recently, orbital atherectomy has been proposed to overcome those limitations. The crown of the OA device in contrast to the RA burr orbits around the periphery of the vessel lumen, affecting mainly the inner parts of the vessel. Additionally, orbital movement reduces obstruction of blood flow through a stenotic lesion and allows for microparticle flushing, reducing the probability of ischemia, slow/flow phenomena, or thermal injury [8]. The crown of the OA device can move at three different speeds: The Glide Assist mode (5000 rpm) is designed to facilitate burr movement inside the guide catheter or healthy parts of a vessel. The low-speed mode (80 000 rpm) is intended for performing atherectomy in vessel diameters lower the 3.0 mm. On the other hand, the high-speed mode (120 000 rpm) can be used in vessels larger than 3.0 mm. The ability of bidirectional ablation minimizes the possibility of crown entrapment. Additionally, the retrograde ablation direction provides additional stabilization of the catheter-guidewire-burr setup, reducing the probability of burr “jump” through the lesion and increasing safety of the procedure. The unique trackability of the guidewire (Viper Wire), combined with full combability with the 6F guiding catheter set-up, predisposes OA to be a reasonable alternative to RA or intravascular lithotripsy [9]. Our case series, among the first in the literature [4, 5] showed that the OA system can be a safe and effective debulking device in cases of high-risk LM lesions. Therefore, considering that subjects with calcified LM lesions are often under-represented in randomized clinical trials [10–12] and portend higher prognostic risk, there is a strong need for large, multicenter, randomized studies comparing the safety and efficiency of OA to other advanced lesion preparation methods (RA and S-IVL) in terms of LM diseases. Additionally, an initial preprocedural and intraprocedural accurate calcification assessment in intravascular imaging is necessary for adequate evaluation of orbital atherectomy efficiency in terms of LM disease. The lack of such evaluation is the main limitation of the present study.








