Summary
The treatment of choice for severe/symptomatic aortic stenosis is valve replacement. Some patients after aortic valve replacement develop a patient-prosthesis mismatch. Overweight plays a relevant role in development of patient-prosthesis mismatch. Patient-prosthesis mismatch occurs more often after a surgical than a transcatheter procedure.
Introduction
Aortic stenosis (AS) is the most common acquired valvular disease. Its incidence increases with age and stands at 2–7% in people over age 65. The treatment of choice in patients with severe and/or symptomatic AS is surgical aortic valve replacement (SAVR) with a mechanical or biological prosthesis or transcatheter aortic valve implantation (TAVI) [1]. Some patients have an increased pressure gradient on the implanted prosthesis after the procedure, due to a patient-prosthesis mismatch (PPM). Currently, PPM is defined as a valve opening area indexed to the body surface (iEOA) equal to or less than 0.85 cm2/m2, while an iEOA value less than 0.65 cm2/m2 characterizes a severe degree of PPM [2, 3].
Aim
Until now, the frequency of PPM depending on the type of valvular prosthesis used has not been assessed in the real-life Polish population. Such an analysis was performed based on the Krakow registry of patients with aortic valve stenosis (KRAK-AS).
Material and methods
The KRAK-AS registry was conducted from July to October 2016 in echocardiography laboratories of departments and outpatient clinics. The study was performed after obtaining a positive opinion from the Ethics Committee of the Jagiellonian University Collegium Medicum in Krakow (ethic statement number: 122.6120.66.2015 from 30th April 2015). The study was conducted in accordance with the principles set forth in the Helsinki Declaration. The data of all consecutive patients with moderate or severe AS, examined during this period, who gave informed consent to participate in the registry were analyzed. The purpose of this prospective registry was to determine the number of patients diagnosed with AS, and their clinical and hemodynamic characteristics, as well as to assess the course of their treatment in a 3-year follow-up. Amongst patients included in the registry, those who had implanted a mechanical valve, a stented biological prosthesis or a TAVI valve were chosen for this analysis. Patients who underwent aortic valve intervention were again clinically and echocardiographically evaluated within a month after surgery by transthoracic echocardiography. Age, gender, body mass index (BMI), calculated body surface area (BSA), prosthesis diameter, maximal and mean transvalvular gradient and indexed effective orifice area were compared between groups. The left ventricular outflow tract (LVOT) dimensions were measured initially by a 2D model, and the effective orifice area (EOA) was calculated nominally. Subgroup analysis of patients with a smaller (< 23 mm) and equal or larger than median (≥ 23 mm) diameter of the valve ring was performed.
Results
The 397 patients with at least moderate aortic stenosis (aortic valve area (AVA) <1.5 cm2 and mean gradient > 20 mm Hg) were included in the registry. Severe stenosis (aortic valve area indexed to body surface area (AVAI) < 0.6 cm2/m2 and/or mean gradient > 40 mm Hg) was found in 288 (72.5%) patients. A hundred and nine (27.5%) patients had moderate stenosis (AVAI between 0.6 and 0.8 cm2/m2). Amongst AS patients, in 3-year follow-up, the valve surgery was performed in 236 patients: the native valve was replaced with a mechanical valve in 42 patients, the biological valve was implanted in 139 and the TAVI procedure was performed in 48 patients. In addition, only balloon aortic valvuloplasty (BAV) was performed in 7 patients – this group was not analyzed in this study.
Mechanical valves were implanted in younger patients (median age: 63.5 years), while the patients who underwent TAVI were older (81 years old). The most frequently implanted mechanical valve was the St. Jude Medical valve (33 patients; 78.6%). Less often the Medtronic ATS valve was implanted (9 patients; 21.4%). In 10 (23.8%) patients the valve replacement was accompanied by replacement of the ascending aorta (Bentall de Bono procedure). The most frequently implanted biological prosthesis was a Carpentier Edwards Perimount valve (68 patients; 48.9%), followed by Sorin Crown (52 patients; 37.4%), less often St Jude Medical Trifecta (15 patients; 10.8%) and Dokimos Plus (4 patients; 2.9%). During the TAVI procedure, Edwards Sapien 3 (20 patients; 41.7%) was the most common implanted valve, followed by Medtronic CoreValve Evolut R (14 patients; 29.1%), Symetis Acurate Neo (8 patients; 16.7%) and Lotus (6 patients; 12.5%) valves. Cardiac surgery procedures were performed mainly in men, while predominantly women received the TAVI valves; however, this difference was not significant (Table I). In all groups the median BMI exceeded the normal value, but only in patients with mechanical and TAVI valves was this difference statistically significant. Similar differences were observed in terms of BSA (Table I). There were no significant differences between subgroups in the left ventricle end-diastolic dimension (LVEDD) and left ventricular ejection fraction (LVEF). Left ventricular mass index (LVMI) and relative wall thickness (RWT) were calculated and showed left ventricular hypertrophy among 38% of patients with a mechanical aortic valve, 27% of patients after biological valve implantation and 35% of patients after a TAVI procedure.
Table I
Demographic and clinical characteristics of patients undergoing aortic valve surgery
| Parameter | Mechanical prosthesis group n = 42 | Biological prosthesis group n = 139 | TAVI Prosthesis group n = 48 | P-value (mech. vs. biol.) | P-value (mech. vs. TAVI) | P-value (biol. vs. TAVI) |
|---|---|---|---|---|---|---|
| Age [years] | 63.5 (54;69) | 70 (65;76) | 81 (78;84) | < 0.0001 | < 0.0001 | < 0.0001 |
| Males (%) | 59.5% | 57.6% | 45.8% | NS | NS | NS |
| BMI [kg/m2] | 28.7 (26.2;34.5) | 27.7 (25.2;31.3) | 25.7 (24;28) | NS | 0.04 | NS |
| BSA [m2] | 2.0 (1.8;2.1) | 1.9 (1.8;2.0) | 1.8 (1.7;1.9) | NS | 0.03 | NS |
| Valve ring diameter [mm] | 23 (21;23) | 23 (21;23) | 26 (23;28.5) | NS | < 0.0001 | < 0.0001 |
| LVEF (%) | 60 (53.7;65) | 60 (55;65) | 60 (50;63.7) | NS | NS | NS |
| LVEDD [mm] | 52 (46;58) | 48 (45;54) | 48 (43;52) | NS | NS | NS |
| Max. trans-prosthetic gradient [mm Hg] | 30.5 (23;53) | 25 (19;32.5) | 17 (13;25) | 0.005 | < 0.0001 | < 0.0001 |
| Mean trans-prosthetic gradient [mm Hg] | 18 (13;31) | 15 (11;20) | 10 (9;13) | 0.01 | < 0.0001 | < 0.0001 |
| iEOA [cm2/m2] | 0.76 (0.66;0.87) | 0.79 (0.71;0.86) | 0.94 (0.87;1.04) | NS | 0.0009 | < 0.0001 |
| EOA [cm2] | 1.52 (1.38;1.52) | 1.5 (1.3;1.5) | 1.74 (1.51;1.74) | 0.004 | 0.004 | < 0.0001 |
| PPM* (% patients) | 17 (40%) | 46 (33%) | 5 (10%) | NS | 0.0001 | < 0.0001 |
| Severe PPM** number (percentage of the valve type group) | 3 (7.1%) | 3 (2.1%) | 0 (0%) | NS | NS | NS |
BMI – body mass index, BSA – body surface area, EOA – effective orifice area, iEOA – effective orifice area indexed to the body surface, LVEDD – left ventricle end-diastolic dimension, LVEF – left ventricular ejection fraction, PPM – patient-prosthesis mismatch, TAVI – transcatheter aortic valve implantation.
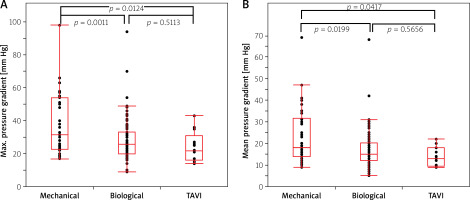
The study showed a statistically significant difference in postoperative transvalvular gradient (both maximal and mean) between the implanted biological prostheses and TAVI valves in the group of patients with a ring diameter of 23 mm or larger. In the group of patients with a smaller valve ring diameter (< 23 mm) a significant gradient difference was found between the mechanical and TAVI valves and between the mechanical and biological valves (Figures 1 A, B, 2 A, B). Both groups of patients with larger and smaller than the median diameter of the implanted valve had lower transvalvular gradients after TAVI compared to SAVR (Table I).
Figure 1
A – Maximal postoperative gradient in groups of patients with different types of prostheses with a ring diameter equal to or greater than 23 mm. B – Mean postoperative gradient in groups of patients with different types of prostheses with a ring diameter equal to or greater than 23 mm
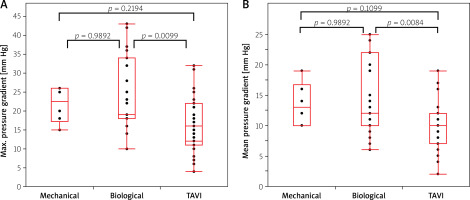
Figure 2
A – Maximal postoperative gradient in groups of patients with different types of prostheses with a ring diameter less than 23 mm. B – Mean postoperative gradient in groups of patients with different types of prostheses with a ring diameter less than 23 mm
The percentage of patients diagnosed with PPM was significantly lower after a TAVI procedure (10%) compared to a mechanical valve (40%), p = 0.0001, and to a biological valve (33%), p < 0.0001. It was comparable in people after mechanical (40%) and biological (33%) valve implantation (Table I). The groups of patients that were compared by means of post-procedural occurrence of PPM were not significantly different at baseline. The native annulus dimensions were comparable in all groups (median diameter was 23.5 mm (21;24) in patients before mechanical valve implantation, 23 mm (21;26) before biological and 23 mm (21;24.75) before TAVI), LVOT diameter was 22 mm (21;24), 21.75 mm (20.6;23.4) and 20.5 mm (18;22) respectively. LVOT VTI (left ventricular outflow tract velocity time) was 24.4 (19.7;28.6), 24.35 (19.5;27.5) and 23.7 (20.4;26.9) respectively. Bicuspid aortic valve was identified pre-procedurally in 21.4% of patients with a mechanical aortic valve, in 16.5% of patients with a biological aortic valve and 6.2% of patients who underwent TAVI. The number of aortic leaflets was not always identifiable.
Amongst 17 patients with PPM after mechanical valve implantation: 3 (17.6%) had a severe form of PPM (iEOA < 0.65 cm2/m2). Amongst 46 recipients of biological valves who had PPM, 3 (6.5%) met the criterion of severe PPM, while no patient had severe PPM after a TAVI procedure. All patients with severe PPM were overweight or obese. The mean prosthetic valve ring diameter was 22 mm and was comparable in both AVR groups. The postoperative gradient on the mechanical valve was higher than on the biological valve despite the lack of statistically significant differences in the mean EOA and iEOA.
Left ventricle overload is of particular importance in patients with severe PPM, among which 3 out of 5 had a maximal transvalvular gradient exceeding 45 mm Hg and a mean pressure gradient higher than 30 mm Hg. It constituted only 1% of all 229 patients treated.
Discussion
Patient-prosthesis mismatch was defined for the first time in 1978 by Rahimtoola as a condition in which EOA of the implanted valve is smaller than physiological [4]. In consequence, increased valve resistance causes increased left ventricular afterload. Hence, patients experience less than expected clinical improvement after the surgery. They may have reduced exercise tolerance, less tendency to postoperative regression of left ventricular hypertrophy, increased postoperative cardiac events and a worse long-term prognosis [5–8]. PPM is also associated with the risk of accelerated progression of the degenerative process of the biological valve. PPM may result in the need for earlier reoperation compared to patients in whom the larger size of the prosthesis provides greater tolerance of its gradual degeneration.
The present results show that PPM defined as iEOA ≤ 0.85 cm2/m2 is not uncommon (29.7% of all analyzed patients and 40% of patients with mechanical valves). Fortunately, severe PPM (iEOA < 0.65 cm2/m2) was observed less frequently – in 2.6% of all operated patients. The moderate form (iEOA 0.65–0.85 cm2/m2) affected 27.1% of patients. This problem occurred mainly in patients with a smaller diameter of the native valve ring (< 23 mm) with surgically implanted mechanical prostheses or stented biological valves. A ring of such prostheses is a necessary element of its construction – it allows the valve to be sewn into the aortic orifice. Nevertheless, it is about 4 mm thick and significantly restricts the valve’s flow area. A significant percentage of moderate PPM also resulted from overweight or obesity [9, 10]. The median BMI in patients with PPM was 27 kg/m2 and the median BSA was 1.86 m2. 41% of PPM patients were overweight, 31% were obese and only every fourth patient had normal body weight.
In contrast to SAVR, the TAVI valve is expanded in the orifice (on a balloon or using the self-expanding method) and has a very small profile of the valve stent. Probably for this reason patients after TAVI have significantly lower transvalvular gradients compared to classically operated patients. A lower gradient could result from a lower flow volume. Therefore, we assessed the size of the left ventricle, the ejection fraction and indexed left ventricular stroke volume and we did not find differences between the valve groups. Lower gradients could also be due to lower BMI and BSA values in the TAVI group, but the difference was borderline (mechanical valve: BMI 28.7 kg/m2, BSA 2.0 m2; biological valve: BMI 27.77 kg/m2, BSA 1.9 m2; TAVI: 25.77 kg/m2, BSA 1.8 m2) and probably was not the decisive element.
On the other hand, low flow severe aortic stenosis was observed before the procedure among: 2.4% of patients treated with a mechanical valve, 3.6% of patients who received a biological prosthesis and 14.6% in whom TAVI was used. This entity may have some influence on pressure gradients, but the above subgroups were rather small and it probably did not impact the results significantly.
The results are consistent with reports from the available literature. Similar conclusions were drawn on the basis of an extensive meta-analysis, which demonstrated a statistically significant reduction in the incidence of PPM after TAVI compared to cardiac surgery, either moderate or severe condition, and total PPM [11–17]. Leonardo Guimaraes et al. observed a lower mean transvalvular gradient, a higher EOA value and a lower index of severe PPM after TAVI compared to SAVR [18]. Based on the data obtained from our registry, similar conclusions can be drawn, but what distinguishes these two studies is the fact that Leonardo Guimaraes et al. focused in their study on patients with a small diameter of the aortic ring (≤ 21 mm) while the present registry contains an analysis of all patients with diagnosed aortic stenosis in a given period regardless of the diameter of the aortic ring. However, these results differ from THE PARTNER 3 study, in which echocardiographic parameters were compared in patients undergoing SAVR and TAVI procedures. It was concluded that transvalvular gradients, effective valve opening area and the incidence of severe PPM were comparable in both groups [19]. It should be noted that only patients with low surgical risk were included in the PARTNER 3 study. In addition, only one type of TAVI valve (Edwards Sapien 3) was taken into consideration in that research.
The difference in the age distribution of patients operated on due to AS has practical reasons: the mechanical valve is potentially the most durable; therefore it is most often implanted in younger people to reduce the frequency of reoperations. In turn, in our registry TAVI was primarily for elderly patients, in whom cardiac surgery would be associated with high perioperative risk due to age and very frequent comorbidities.
An important aspect is the fact that the analysis was conducted on a nonselected group of consecutive real life AS patients examined in a selected period of 4 months during which this registry was conducted. It realistically reflects the characteristics of the Polish population with AS, who subsequently underwent currently available invasive procedures.
Study limitations: The analysis is based on a prospective registry and not on a randomized trial, which would more purely methodically compare hemodynamics of individual valves. Randomization, however, is not possible for ethical reasons. The qualification for particular types of surgery was based on the age of the patients (younger patients received the most durable mechanical valves in accordance with the guidelines) and the overall clinical assessment (older patients received biological prostheses and those with high operational risk were qualified for TAVI). The registry included echocardiographic examination performed in various laboratories; hence the possibility of slight methodological differences exists. The study assessed the valve soon after surgery, and evaluation of the prognostic significance of the PPM phenomenon is not possible due to the limited group size and insufficient systematic long-term follow-up.
Conclusions
Patients after a TAVI procedure have lower transvalvular gradients compared to patients with implanted mechanical or biological valves. The percentage of patients with a moderate degree of PPM is high in the real-life Polish AS population and is significantly higher in patients undergoing SAVR than in patients after TAVI. The incidence of severe PPM is low, and it occurs mostly in SAVR rather than in TAVI patients. The incidence of PPM was comparable in the groups with implanted mechanical and biological valve prostheses.








