Introduction
Hidradenitis suppurativa (HS, acne inversa) is a chronic disease characterized by recurrent, painful, deep-seated nodules and abscesses of apocrine gland-bearing skin [1]. HS causes painful discomfort as well as social embarrassment, which has a negative influence on the quality of life [2]. A mean disease incidence of 6.0 per 100,000 person-years and an average prevalence of 1% has been reported in Europe [3]. The first reports about HS in the literature were published in 1839 by Valpeau [4]. Acne inversa (HS) also known as Hidradenitis Suppurativa or Verneuil’s Disease is an inflammatory chronic disease of the hair follicle that presents with different lesions in the apocrine gland-bearing areas of the human body. Most common areas are axillae, inguinal and anogenital regions [4]. Presentation of the disease involves a classic distribution of painful lesions in the skin creases with associated local complications of abscesses and sinuses [5]. There are often systemic symptoms, and management is handled by various specialties such as dermatology, general surgery or plastic surgery. Hidradenitis suppurativa is multispecialty disease. As a result of this multidisciplinary approach to the disease, an accurate diagnosis is often delayed. The exact aetiology remains unknown, but it is suggested to be associated with smoking, diabetes, poor hygiene, immunocompromised and reduced cutaneous levels of calprotectin, zinc, or ascorbate. Hidradenitis suppurativa is associated with several comorbidities, including diabetes mellitus and Crohn’s disease. The clinical presentation of hidradenitis suppurativa ranges from rare, mild inflammatory nodules to widespread abscesses, sinus tracts, and scarring The primary mechanism is a follicular occlusion with secondary inflammation, and destruction of the pilo-sebaceous-apocrine apparatus and extension to the adjacent subcutaneous tissue. It resulted in an inflammatory response that is associated with bacterial infection of the apocrine sweat glands [6]. Prevalence of HS has been shown to be greater in females than males [7]. Cutaneous squamous cell carcinoma (SCC) is the second most common non-melanoma skin cancer. It accounts for 20% of skin cancer cases [8]. Squamous cell carcinomas also known as epidermoid carcinomas, comprise a number of different types of cancer that result from squamous cells [9]. These cells are formed on the surface of the skin, on the lining of hollow organs in the body, and on the lining of the respiratory and digestive tracts [10].
The literature has documented SCC as a rare complication of chronic HS lesions. SCC development within pre-existing HS lesions has been considered to be the most severe complication of HS. Despite the extensive documentation of cases detailing SCC complicating HS lesions, the relationship between HS lesions and SCC is yet to be explored and integrated [8–10]. Early recognition of SCC in an HS patient is very important for treatment.
Aim
The performed a literature review with the aim of identifying and summarizing the characteristics, and meta-analysis of current studies in this field.
Material and methods
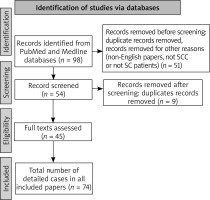
The study identification on 16 December 2021, the PubMed, Medline databases were searched using various forms of two key words: “hidradenitis suppurativa” and “squamous cell carcinoma”. We also used alternative forms of these key words: “HS”, “SCC” and “acne inversa”. The publication dates we chose were between 1900 and 2021 because most of the medical literature about Hidradenitis Suppurativa was published after 1950 (Hurley). We found 98 papers (abstracts, original texts, case reports). We excluded 53 papers. This literature review included original articles only written in the English language, study population (HS patients with SCC), case series, case reports or experimental randomized controlled trials. The analysis focused on the general characteristics of the patient (age, sex, potential risk factors such as addictions, HPV infection), on the side of characteristics of the patients with HS (age, duration of HS, Hurley Stage, location of the lesions, treatment), on the side of characteristics of patients with SCC (age at the time of diagnosis, location of SCC, stage of malignancy, metastasis, cancer treatment, mortality rate). We included 74 patients with HS and SCC. The flowchart of study selection is presented in Figure 1. The oldest case of SCC in HS was from 1964. Literature included in this review comprises 45 original papers [10–54].
Results
All the case reports and series met minimum quality assessment standards. The identification of the data was very difficult, due to their rarity. Most of the papers were case reports, and authors had different criteria in the assessment of each case. First of all, we focused on patient demographics and general characteristics and then we examined SCC data (SCC characteristics). While a few authors did not elaborate on the histological details, patient details, and the attempted treatments, overall methodological quality of the selected articles was found to be satisfactory.
HS patients general characteristics
A total of 74 cases were included in this analysis. General HS patients characteristics are detailed in Table 1. The majority was male patients – 85.1% (63 male cases vs. 11 female cases). The mean age of the patients was 52.67 years. HS duration time (mean) was 25.79 years. Most patients were in Hurley Stage III (Table 1). We also focused on risk factors, which could potentially play a role in neoplastic processes. Risk factors such as smoking, obesity, HPV infection, HIV, Crohn’s disease were included in our analysis. Only 5 patients were reported to be using immunosuppressive systemic therapy for HS.
Table 1
Hidradenitis suppurativa patients profile
SCC general characteristics
We analysed the location of SCC on the HS lesions. The majority was located in the gluteal/perianal region (94.59%). The most common morphology was tumour and ulcer on the HS lesion. We also focused on metastatic status where we found that 62.8% of included patients have no metastatic data. We also took into consideration the mortality rates and no recurrence rate with follow-up consultation with the patients (6 months, 12 months, 18 months, 24 months, 36 months, 4 years, 25 years). General characteristics of SCC in HS patients are shown in Table 2.
Table 2
General characteristics of SCC
Associations/risk factors
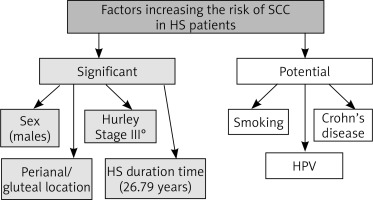
The average age of the patient with SCC in HS lesions was 52.6 years. The mean time from onset of HS to SCC was 25.79 years (range 8 years to 53 years). Majority of the patients was in Hurley Stage III (97.2%). The most frequent region where SCC was located on the HS lesion was perianal/gluteal area (94.59%). Patients rarely presented with HS in the inguinal and axillary region 36% of the patients were HPV positive and 24% were smokers. 12% were diabetic, and 12% also had Crohn’s disease. We divided risk factors that increase the risk of SCC in HS to a) significant and b) potential which is presented in Figure 2.
Discussion
SCC is the second most common skin cancer [8]. The most important risk factors for the development of SCC are sun exposure, age and type of the skin. It is also described in chronic wounds [55–61]. Immunosuppression is one of the risk factors. Some of the authors described mutation in the suppressor protein (TP53) [62]. Pathogenesis of SCC arising in the sun exposed regions seems to be improper in HS patients. Most of the lesions are located in the gluteal and perianal region. The development of SCC is multifactorial. HS is more prevalent in women, but SCC transformation is more frequent in men [15, 17]. SCC in HS is frequently located in the perineal and gluteal areas. These areas are exposed to bacterial, fungal, or viral infections and could promote chronic inflammation and thus result in the occurrence of SCC. HPV was present in 8 genital or anal tumours; among them, HPV-16 (high risk) was present in 7 cases. HPV may be an important contributory factor to SCC arising in HS. Of the 74 published cases analysed in this study, SCC almost always occurred in males with Hurley Stage III and long-standing gluteal/perianal HS. Evidence of HPV was demonstrated in 36% of tested patients.
The nonavalent HPV vaccine (®Gardasil-9) is approved for the prevention of HPV-related mucosal cancers in women ≤ 45 and men ≤ 26 years of age, and there is no doubt that should be considered as a prevention of SCC [63]. Flores et al. noted a significant positive correlation of HPV-16 viral load between proximal anatomic sites in the anogenital region of men, suggesting a possible autoinoculation in male HPV HS patients, facilitated by humidity and inadequate hygiene due to chronic pain in the affected area [64]. Immunosuppressive therapies can possibly cause cancers, but in our analysis, we found only 5 cases with such a therapy. Smoking is also a potential risk factor which often occurs in SCC HS patients.
Conclusions
We suggest that the most significant risk factors in HS patients are sex (males), long HS duration time, perianal/gluteal region, and Hurley Stage III. Considering the high mortality rate (40%), we suggest screening/examining SCC lesions in all HS patients for SCC. The potential risk factors such as males, Hurley Stage III, long HS duration time and location of HS in the perianal/gluteal region should be considered. As HS is a chronic skin disease with the possibility of SCC occurring in these lesions, we should be encouraged to adopt surgical rather than conservative dermatological treatment.