Summary
Patients after coronary revascularization procedures before transcatheter aortic valve implantation (TAVI) constitute a high-risk population. As the complexity of coronary artery disease increases, the beneficial effect of percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) prior to TAVI may be counterbalanced in severe aortic stenosis by increasing risk of periprocedural complications. This study demonstrated no significant difference in periprocedural and 1-year mortality between revascularized and non-revascularized patients prior to TAVI. However, post-TAVI stroke rate is increased in patients after PCI or CABG. In the Cox proportional-hazards regression model, acute kidney injury stage 2 and 3 and post-TAVI stroke were independently correlated with 1-year mortality. TAVI seems to be a safe and effective procedure for the treatment of severe aortic stenosis in patients with previous coronary revascularization.
Introduction
Prevalence of coronary artery disease (CAD) in patients with aortic stenosis (AS) is high, ranging from 30% to 75% [1–5]. Both conditions share pathogenesis, risk factors and symptoms, but the latter tend to occur earlier in CAD.
Transcatheter aortic valve implantation (TAVI) is a recommended treatment procedure for patients ≥ 75 years with severe AS. It has been demonstrated that it is not inferior to surgical aortic valve replacement (SAVR) in intermediate, high-risk and inoperable patients with severe AS [6–10].
The wide range of CAD incidence in TAVI patients results from differences in the assessment of the severity of atherosclerosis in coronary arteries and the occurrence of myocardial ischemia. This is directly related to the difficulties in determining the risk of ischemic events and mortality after TAVI in this subgroup of patients.
TAVI is most often regarded as a stand-alone procedure, with varying degrees of co-occurring CAD well tolerated without any invasive treatment. Previous coronary revascularization, either with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), can be used as a marker for the presence of coexisting CAD. Patients after coronary revascularization procedures before TAVI constitute a high-risk population. SAVR in CABG patients increases operative risk due to the risk of bypass damage and inadequate protection of myocardial ischemia during cardiopulmonary bypass; therefore, TAVI has emerged as an alternative option for these patients in recent years [11, 12]. As CAD becomes more complex, the positive effect of PCI or CABG prior to TAVI may be counterbalanced in severe aortic stenosis by increasing risk of periprocedural complications due to left ventricular hypertrophy, elevated left ventricular pressure and increased contraction force, resulting in intramyocardial compression of the microcirculation [5]. Thus, it is still debatable whether a history of PCI or CABG prior to TAVI increases the risk of a TAVI procedure and influences early and mid-term outcomes.
Aim
The aim of this study was to evaluate the impact of previous coronary revascularization in terms of PCI or CABG on clinical outcomes after TAVI.
Material and methods
For the purpose of the study we included 507 consecutive patients who received TAVI from March 2010 to July 2019 and who gave their informed consent to enter the Transcatheter Valve Treatment Sentinel Registry (2010–2012), which later was continued by the national POL-TAVI registry database [13, 14]. All patients were diagnosed with severe symptomatic aortic stenosis (aortic valve area < 1.0 cm2 or indexed valve area < 0.6 cm2/m2 or mean gradient > 40 mm Hg or maximum jet velocity > 4.0 m/s or velocity ratio < 0.25), and after the heart team decision underwent TAVI.
The study population was divided into three groups based on the history of previous coronary revascularization: non-revascularization (NR), post-PCI and post-CABG. PCI or CABG prior to TAVI was defined as occurring at any time before TAVI, including patients undergoing PCI as part of pre-TAVI management. PCI occurring during the referral process for TAVI was performed within 4 weeks prior to TAVI as a staged procedure.
Data related to patients’ characteristics and periprocedural outcomes were collected prospectively. Patients with an unconfirmed status were followed up remotely by telephone visits. All patients completed a 12-month follow-up, as obligated by the registry protocol. Informed consent was obtained from all participating patients and the local ethics committee granted permission for the study. All definitions are in accordance with the Valve Academic Research Consortium-2 consensus document [15].
Statistical analysis
The Shapiro-Wilk test was used to confirm or reject the normal distribution of each continuous variable. Continuous variables with normal distribution were presented as the mean with standard deviation (SD). Continuous variables with non-normal distribution were presented as the median with interquartile range (IQR) and compared with the Kruskal-Wallis test. Categorical variables, expressed as counts and percentages, were compared using the χ2 test or Fisher’s exact test, as appropriate.
Kaplan-Meier curves and log-rank tests of the time-to-event data were used to assess the differences of 30-day and 1-year all-cause mortality between the groups. Cox proportional hazard analysis was performed to find possible predictors of mortality in 30-day and 1-year observation for all groups. All probability values reported are 2-sided and a value < 0.05 was considered to be significant. The data were analyzed using MedCalc ver. 21 (MedCalc Software Ltd, Ostend, Belgium, www.medcalc.org).
Results
After being divided into subgroups, the non-revascularization (NR) population consisted of 321 (63.3%) patients, PCI prior to TAVI was performed in 127 (25%) patients and CABG prior to TAVI in 59 (11.7%) patients. Taken together, patients with a history of previous revascularization (PCI + CABG) constituted 186 (36.7%) of the study group. Comparison of baseline characteristics between the groups demonstrated significant differences in terms of either median age and percentage of the female sex (Table I). In the NR and post-PCI groups, the patients were older (NR = 81 (76–84) vs. post-PCI = 82 (82–83) vs. post-CABG = 77 (75–82); p = 0.004) and there were more women (NR = 59.5% vs. post-PCI = 42.5% vs. post-CABG = 25.4%; p < 0.0001) in comparison to the post-CABG group. There was a clear difference in EuroSCORE II values with CABG patients being at the highest risk (NR = 7.86 (4.77–16.64) vs. PCI = 8.02 (4.78–19.10) vs. CABG = 20.5 (8.33–28.55); p < 0.0001). Patients after CABG also had higher rates of diabetes, chronic kidney disease, chronic obstructive pulmonary disease, history of myocardial infarction, and peripheral artery disease (Table I).
Table I
Baseline characteristics
[i] AVA – aortic valve area, BMI – body mass index, CABG – coronary artery bypass grafting, CKD – chronic kidney disease, COPD – chronic obstructive pulmonary disease, EF – ejection fraction, IQR – interquartile range, MI – myocardial infarction, NR – non-revascularization, PAD – peripheral artery disease, PCI – percutaneous coronary intervention, PG – pressure gradient.
There were significant differences in baseline aortic valve area (AVA), mean pressure gradient (PG) and left ventricular ejection fraction (EF) before TAVI (Table I). The post-CABG group was characterized by the lowest EF (NR = 58 (48–65)% vs. post-PCI = 55 (40–60)% vs. post-CABG = 53 (41–60)%; p < 0.01) and mean PG (NR = 45 (34–55) mm Hg vs. post-PCI = 44 (37.50–50.25) mm Hg vs. post-CABG = 41 (28.75–46.25) mm Hg; p < 0.01).
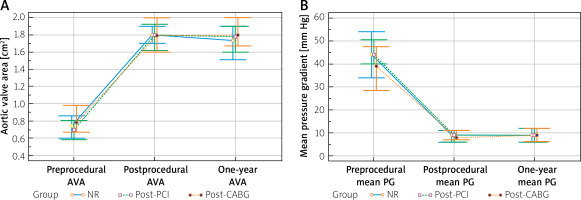
All patients achieved significant postprocedural improvement in the aortic valve area and mean PG, which was sustained in 1-year observation. There were no between-group differences in incidence of moderate to severe paravalvular leakage. No significant differences in echocardiographic parameters between groups were observed during the postprocedural follow-up (Figure 1).
Figure 1
Comparison of aortic valve area (A) and mean pressure gradients (B). Clustered multiple variable graphs of aortic valve area (AVA) and mean pressure gradients (mean PG) presented as median with interquartile range. Preprocedural AVA: NR 0.75 (0.60–0.85) cm2 vs. post-PCI 0.68 (0.56–0.80) cm2 vs. post-CABG 0.81 (0.67–0.94) cm2; p < 0.001. Postprocedural AVA: NR 1.8 (1.6–1.9) cm2 vs. post-PCI 1.8 (1.6–2.0) cm2 vs. post-CABG 1.8 (1.6–2.0) cm2; p = 0.85. 1-year AVA: NR 1.7 (1.5–1.9) cm2 vs. post-PCI 1.8 (1.6–1.9) cm2 vs. post-CABG 1.8 (1.6–2.0) cm2; p = 0.67. Preprocedural mean PG: NR 45 (34–55) mm Hg vs. post-PCI 44 (38–50) mm Hg vs. post-CABG 41 (29–46) mm Hg; p < 0.01. Postprocedural mean PG: NR 9 (6–11) mm Hg vs. post-PCI 8 (6–11) mm Hg vs. post-CABG 7 (6–11) mm Hg; p = 0.54. One-year mean PG: NR 9 (6–12) mm Hg vs. post-PCI 9 (6–11) mm Hg vs. post-CABG 9 (7–12) mm Hg; p = 0.72
NR – non-revascularization, PCI – percutaneous coronary intervention, CABG – coronary artery bypass grafting.
Procedural details are presented in Table II. A Sapien XT bioprosthesis was used more often in the post-CABG group whereas the Portico bioprosthesis was implanted significantly more often in the post-PCI group. Approximately 80% of TAVIs were performed via the transfemoral approach, and other vascular approaches were more frequently used in patients after coronary revascularization. Balloon predilation was performed less frequently in patients after CABG, while the frequency of postdilation did not differ between the groups. There were no significant differences in procedure time, contrast volume and radiation dose between study groups.
Table II
Procedural characteristics
| Variable | All (n = 507) | NR (n = 321) | Post-PCI (n = 127) | Post-CABG (n = 59) | P-value |
|---|---|---|---|---|---|
| Prosthesis type, n (%): | |||||
| CoreValve | 114 (22.5) | 75 (23.3) | 30 (23.6) | 9 (15.2) | 0.37 |
| Sapien XT | 70 (13.8) | 43 (13.4) | 12 (9.5) | 15 (25.4) | 0.013 |
| Sapien 3 | 9 (1.8) | 7 (2.2) | 2 (1.6) | 0 (0) | 0.50 |
| Lotus | 34 (6.7) | 24 (7.5) | 5 (3.9) | 5 (8.5) | 0.34 |
| Evolut R | 143 (28.2) | 87 (27.1) | 34 (26.8) | 22 (37.3) | 0.26 |
| Evolut PRO | 26 (5.1) | 20 (6.2) | 4 (3.1) | 2 (3.4) | 0.34 |
| Portico | 104 (20.5) | 60 (18.7) | 39 (30.7) | 5 (8.5) | < 0.001 |
| Othera | 7 (1.4) | 5 (1.6) | 1 (0.8) | 1 (1.7) | 0.80 |
| Access, n (%): | |||||
| Femoral | 429 (84.6) | 279 (86.9) | 102 (80.3) | 48 (81.3) | 0.17 |
| Subclavian | 19 (3.8) | 7 (2.2) | 11 (8.7) | 1 (1.7) | 0.004 |
| Carotid | 25 (4.9) | 12 (3.7) | 8 (6.3) | 5 (8.5) | 0.22 |
| Transapical | 9 (1.8) | 5 (1.6) | 0 (0) | 4 (6.8) | 0.004 |
| Direct aortic | 25 (4.9) | 18 (5.6) | 6 (4.7) | 1 (1.7) | 0.44 |
| Procedure, n (%): | |||||
| Predilatation | 353 (69.6) | 233 (72.6) | 91 (71.7) | 29 (49.2) | 0.001 |
| Postdilatation | 166 (32.7) | 107 (33.3) | 47 (37.0) | 12 (20.3) | 0.07 |
| Valve-in-valve | 8 (1.6) | 3 (1.0) | 2 (1.6) | 3 (5.1) | 0.06 |
| Procedure timeb, median (IQR) [min] | 180 (150–220) | 185 (150–220) | 180 (150–228) | 180 (150–204) | 0.76 |
| Contrast, median (IQR) [ml] | 200 (15–250) | 200 (160–245) | 200 (150–250) | 200 (150–254) | 0.87 |
| Radiation dose, median (IQR) [mGy] | 1278 (854–2104) | 1258 (772–2047) | 1268 (960–2220) | 1377 (939–2324) | 0.07 |
| Access closure, n (%): | |||||
| Percutaneous | 351 (69.2) | 226 (70.4) | 89 (70.1) | 36 (61.0) | 0.35 |
| Surgical | 156 (30.8) | 95 (29.6) | 38 (29.9) | 23 (39.0) | 0.35 |
A comparable length of hospital stay was observed in NR, post-PCI and post-CABG groups (15.0 (9.00–22.00) vs. 13.0 (8.00–20.25) vs. 14.0 (10.00–22.00) days; p = 0.21).
The rates of postprocedural complications were similar between study groups, both at 30-day and 1-year follow-up, except for the incidence of stroke, which was significantly lower in NR patients as compared with patients after coronary revascularization (Tables III and IV). Patients after PCI or CABG prior to TAVI had similar 30-day all-cause mortality rates as those without coronary revascularization at baseline (NR vs. post-PCI vs. post-CABG: 8.1% vs. 5.5% vs. 6.8%, respectively; p = 0.6) (Table III).
Table III
Clinical outcomes at 30-day follow-up
Table IV
Clinical outcomes at 1-year-follow-up
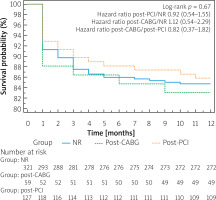
There were no differences in 12-month all-cause mortality rates between groups (NR vs. post-PCI vs. post-CABG: 15.3% vs. 14.2% vs. 16.9%, respectively; log-rank p = 0.67) (Figure 2).
Figure 2
One-year survival after transcatheter aortic valve implantation, according to the history of coronary revascularization prior to TAVI. Kaplan-Meier survival curves after TAVI in NR (blue full line), post-CABG (dotted green line) and post-PCI (dotted yellow line) subgroups
NR – non-revascularization, PCI – percutaneous coronary intervention, CABG – coronary artery bypass grafting.
No influence of NR (for 30-day mortality HR = 1.01, 95% CI: 0.55–1.87, p = 0.96; for 12-month mortality HR 1.01, 95% CI: 0.64–1.62, p = 0.94), PCI (for 30-day mortality HR = 0.8, 95% CI: 0.38–1.72, p = 0.59; for 12-month mortality HR = 0.92, 95% CI: 0.54–1.58, p = 0.76) or CABG (for 30-day mortality HR = 1.36, 95% CI: 0.59–3.11, p = 0.47; for 12-month mortality HR = 1.12, 95% CI: 0.57–2.2, p = 0.75) on the risk of death was found.
In the Cox proportional-hazards regression model, acute kidney injury stage 2–3 (HR = 6.05, 95% CI: 1.5–24.7, p = 0.01) and EF (HR = 0.93, 95% CI: 0.9–1.0, p = 0.002) were identified as independent predictors of mortality at 30 days after TAVI, and acute kidney injury stage 2–3 (HR = 3.7, 95% CI: 2.14–6.33; p < 0.001) and post-TAVI stroke (HR = 3.5, 95% CI: 1.57–7.8; p = 0.002) were independently correlated with 1-year mortality.
Discussion
Our study confirmed that previous coronary revascularization is frequent among high-risk AS patients. Approximately 37% of the patients were after PCI or CABG at the time of pre-TAVI work-up, which puts our data well within the already reported incidence range [16–19].
The patients after PCI or CABG are distinctly a higher-risk cohort, as has been shown by the higher logistic EuroSCORE and lower ejection fraction. For almost 10 years of this single-center experience, in conjunction with modifications in TAVI technique and periprocedural management of patients, lower rates of complications and improvement in 1-year survival were achieved [20]. However, the rate of stroke and the need for permanent pacemaker implantation have not been reduced over time [20]. The age of TAVI patients has not changed over the past decade in our study group, and along with an increase in CAD prevalence in elderly patients with severe AS, there are concerns about the safety and efficacy of TAVI in this subset of patients.
Significant hemodynamic improvement, in terms of enlarged aortic valve area, increased ejection fraction, and reduced peak gradients, was observed in all patients after TAVI. To determine whether this translates into increased life expectancy, further studies of low-risk populations are likely to be necessary.
This study revealed that CAD, as defined by previous CABG or PCI, was not a significant risk factor for mortality in patients having TAVI.
We consider this as the most practical finding of this study, demonstrating that this additional risk factor does not affect mortality at 30 days and 1 year. Overall, the present 7.3% TAVI 30-day mortality rate was similar to that published in most other studies and there was no significant difference between CAD and non-CAD patients [21, 22]. Contrary to our findings, Dewey et al. reported a 10-fold higher 30-day mortality rate among CAD compared to non-CAD patients (13.1% vs. 1.2%, p = 0.002) [23]. However, almost 20% of patients in this study underwent transapical TAVI and the logistic EuroSCORE was 35.8 (±15.9)%. Similar to our study, CAD was defined only as previous coronary artery revascularization, which excluded all the patients with CAD treated medically or newly discovered. This definition resulted in the inclusion in the non-revascularization group of all the other subsets of patients with non-obstructive CAD, obstructive CAD, CAD without revascularization and no CAD. This potentially could overestimate the frequency of adverse events in this group. On the other hand, PCI prior to TAVI appears to improve survival to levels comparable to those of patients without co-existing obstructive CAD [18]. Moreover, progression of CAD was excluded in all patients by diagnostic angiography before they were scheduled for TAVI. The presence of medically treated CAD may potentially increase the risk of cardiac ischemia during TAVI, but there is a lack of data on the risk stratification in those patients and no consensus on the optimal diagnostic method in this context [5, 17].
The present data suggest that a history of previous coronary revascularization can be perceived as a type of cumulative risk factor for this heterogeneous population of CAD patients having TAVI. Notably, in the multicenter randomized PARTNER trial, patients with a history of myocardial infarction and those requiring PCI or CABG before TAVI were excluded [24].
There are only a few studies reporting mortality rates in patients with significant CAD undergoing TAVI. Dewey et al. identified CAD as an independent predictor of short- and long-term mortality, but these data were not supported by other studies [23]. A meta-analysis by Sankaramangalam et al. revealed that CAD accompanying TAVI did not affect 30-day mortality, but it had an influence on 1-year mortality and procedural complications were not different regarding CAD status [25]. The inconsistencies resulted from the lack of a consistent definition of CAD, heterogeneous nature of the disease and TAVI outcomes were not stratified by CAD. A meta-analysis by D’Ascenzo et al. demonstrated no influence of CAD on mortality in patients undergoing TAVI [17]. This analysis was constrained by the lack of uniform definition of CAD across multiple pooled studies. Other studies on PCI outcomes and a completeness of revascularization before TAVI have mostly failed to show any reduction in mortality or in major cardiovascular events [3, 26]. According to our data, there were no significant differences between post-PCI or post-CABG and non-revascularized patients in all-cause 30-day mortality rate (5.5% vs. 6.8% vs. 8.1% respectively; p = 0.6). In our study, similar to other studies, revascularization prior to TAVI did not cause a significant difference in 1-year mortality [21]. Paradis et al. in a retrospective analysis of severity of CAD in patients who underwent TAVI concluded that completeness of revascularization, for either PCI or CABG, did not impact the incidence of MI, stroke, and death at 30 days and 1 year [27]. A large meta-analysis found no significant differences in 30-day and 1-year all-cause mortality among TAVI patients with or without a history of previous cardiac surgery with sternotomy (risk ratio 0.95, 95% CI: 0.82–1.09, p = 0.55; risk ratio 0.94, 95% CI: 0.86–1.02, p = 0.48, respectively) [18]. Furthermore, the results of subgroup analysis, including only CABG patients, also did not demonstrate differences among these groups [18]. Ducrocq et al. observed improved survival in patients who had CABG prior to TAVI. A history of CABG was an independent predictor of improved 2-year survival in the multivariate analysis [28]. We observed in this study that post-PCI or post-CABG patients did not have excess mortality 1 year after TAVI, compared to non-revascularized patients (14.2% vs. 16.9% vs. 15.3%, respectively; p = 0.88). Based on our results, we assume that previous coronary revascularization has a limited effect on the procedural risk and mid-term outcome of TAVI.
In spite of a higher incidence of post-TAVI stroke in patients with prior coronary revascularization in our study, the other unfavorable outcome measures did not differ from those of patients with no history of revascularization. Post-TAVI stroke (HR = 3.5, 95% CI: 1.57–7.8; p = 0.002) was independently correlated with 1-year mortality in the whole cohort of our patients but there was no apparent difference between the study groups regarding the impact of stroke on all-cause mortality at 1 year after TAVI. Possible explanations for increased stroke in patients after PCI or CABG include an increased atheromatous burden and release of embolic material from the vasculature during the procedure. Cerebral embolic protection (CEP) devices are designed to trap arterial debris to potentially reduce the risk of stroke. An analysis by the Society of Thoracic Surgeons–American College of Cardiology Transcatheter Valve Therapy Registry demonstrated the use of CEP in 13% of TAVI procedures [29]. Kapadia et al. randomly assigned patients to undergo transfemoral TAVI with CEP or without CEP [19]. The incidence of stroke within 72 h after TAVI or before discharge did not differ significantly between the CEP group and the control group (2.3% vs. 2.9%; p = 0.30), although in subgroup analyses performed according to sex and operative risk, patients with CAD were characterized by a numerically lower rate of stroke after use of CEP (1.8% vs. 3.0%). With reference to the results of our study, perhaps patients after coronary revascularization would be suitable candidates for CEP to reduce the incidence of stroke. The legitimacy of using CEP devices requires a more precise determination of subsets of patients who may benefit from their use.
With the extension of indications for TAVI in the group of low-risk patients with AS, the number of patients treated with this method is expected to increase significantly and TAVI will become a dominant procedure in the treatment of severe AS. At the same time, in the systematically growing population of elderly AS patients with co-existing coronary artery disease, problems related to revascularization of coronary arteries, e.g. the position of an aortic valve prosthesis, access to coronary ostia, valve-in-valve procedures, or paravalvular leak closure, still remain a challenge [30]. Increasing the availability of TAVI procedures, proper risk stratification for decision-making, a patient-tailored approach, and capacity for transcatheter management of valvular procedures complications are the main tasks to be implemented in the near future [30].
The main limitation of our study is its retrospective methodology. The larger population of the study would allow for more complete identification of independent predictors of mortality. Nevertheless, the current dataset is one of the largest in Poland to date. Disadvantages are also due to its single-center design but the fact that our cohort was monocentric enables the assessment of the uniform treatment strategy in relation to outcomes. We were unable to analyze the time from PCI or CABG to TAVI at baseline due to the lack of this information in both the hospital records and the POL-TAVI registry. If the decision to perform PCI was made during the referral process for TAVI, in over 95% of cases PCI was performed within 4 weeks prior to TAVI as a staged procedure, with only a minority of cases performed as a joint procedure on the day of TAVI, immediately before the bioprosthesis deployment. For this reason, and due to inability to independently analyze angiogram results at baseline, we were unable to identify patients with obstructive CAD and not undergoing coronary revascularization before TAVI. These patients were included in the NR group, which potentially may overestimate the frequency of adverse events in this group. The decision for suitability and eligibility for TAVI was conducted by a multidisciplinary local Heart Team according to current guidelines, although potential bias in patients and treatment selection could affect the outcomes.
Conclusions
This study demonstrates no significant difference in periprocedural and 1-year mortality between revascularized and non-revascularized patients prior to TAVI. However, the post-TAVI stroke rate is increased in patients after PCI or CABG. Randomized prospective trials are needed to establish the role of revascularization in patients with significant CAD undergoing TAVI.








