Summary
In our registry-based analysis of propensity-matched patients, patients with a previous medical record of immunosuppressive treatment who had undergone transcatheter aortic valve implantation (TAVI) for aortic stenosis (AS) during almost 3 years after the procedure had comparable mortality to patients without a history of immunosuppression. The follow-up in this study is one of the longest ones available for this specific group of patients. Moreover, severe procedural complications occurred at similar rates in the two groups. These results might help in the difficult decision-making process of Heart Teams selecting patients for AS treatment. Our data show that TAVI can be a viable treatment option for immunosuppression treated patients.
Introduction
Aortic stenosis (AS) is the most common type of heart valve disease in the elderly, with over one in eight people above 75 years of age being diagnosed with AS [1, 2]. Severe, symptomatic AS is a life-threatening condition, with a 50% mortality rate within 2 years after the onset of symptoms [3]. Transcatheter aortic valve implantation (TAVI) is one of the established treatment methods for AS, especially preferred in patients above 75 years of age and/or at high surgical risk of perioperative mortality after surgical valve replacement [4]. The number of TAVI procedures increases annually and the expansion of indications for this method is more than likely [5]. Although TAVI is associated with a satisfactory long-term prognosis, the complications related to TAVI include conduction disturbances, bleeding, stroke or vascular access site complications, which strongly correlate with in-hospital mortality [6]. Patients treated with immunosuppression are at especially high risk of complications when undergoing TAVI due to tissue damage and accelerated atherosclerosis triggered by immunosuppressive drugs. For example, treatment with glucocorticoids is an established risk factor contributing to frailty and bleeding risk, especially in the elderly, which results in an increased risk of vascular complications [7, 8].
Aim
Considering that data concerning TAVI outcomes in the immunosuppressed patients are scarce, we developed this study with the aim of comparing the safety and influence on mortality of TAVI in patients treated with chronic immunosuppression and patients without such treatment.
Material and methods
We conducted a multicentre, propensity-score-matched, registry-based analysis of patients with AS treated with TAVI at 5 experienced academic centres in Poland. The study was formally approved by the Bioethics Committee of the Medical University of Warsaw (approval number AKBE/226/2019). Patients with symptomatic severe AS who underwent TAVI after local Heart Team consultation were included in the study. Heart Teams comprised at least a general cardiologist, an interventional cardiologist, and a cardiac surgeon. Exclusion criteria comprised aborted procedures and previous aortic valve replacement. All participating hospitals used standardized definitions to gather clinical information including patient demographics, laboratory data, comorbidities, procedural details, and in-hospital outcomes. Long-term mortality data were collected from the Polish National Health Service database.
Outcomes
The primary outcome was long-term all-cause mortality after TAVI in patients with chronic immunosuppression compared to patients without it. Secondary outcomes included (i) major vascular complications, (ii) composite endpoint composed of life-threatening or disabling bleeding, major vascular complications, stroke and new pacemaker implantation. All adverse outcomes were defined using Valve Academic Research Consortium-2 (VARC-2) definitions [9].
Statistical analysis
Among all patients included in the registry, we selected patients who received chronic immunosuppressive therapy for at least 30 days before the procedure (study group). The control group was then selected out of patients not treated with immunosuppression using the propensity-score matching procedure in a 1 : 3 ratio, to account for differences in baseline and procedural characteristics between the groups. Several methods of matching patients to the control subjects were evaluated by an independent statistician (K.P.). The propensity score was calculated based on eight characteristics with a previously demonstrated impact on post-TAVI outcomes [10, 11] including age, sex (male), peripheral artery disease, haemoglobin level, estimated glomerular filtration rate, left ventricular ejection fraction (LVEF), access site and valve size. Missing data were not imputed and the quality of matching was assessed by analysing the charts presenting discrepancies in baseline characteristics between groups. Eventually, optimal group matching was conducted using the matchIt R package [12]. Categorical variables were presented as numbers and percentages and were tested for between-group differences using either the χ2 or Fisher’s exact test. Normality of distribution of continuous variables was assessed using the Shapiro-Wilk W test. Normally distributed continuous variables were presented as mean and standard deviation (SD), while those departing from normal distribution were presented as median with interquartile range (IQR). Two-sample t tests or Mann-Whitney U tests were used to test the between-group differences in continuous variables. The long-term mortality rates were analysed using Kaplan-Meier curves, the Cox proportional-hazards model and log-rank test. Only two-sided statistical tests were used and statistical significance was assumed for p < 0.05.
Results
Baseline characteristics
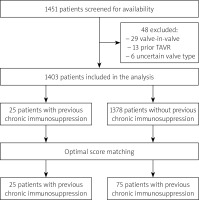
The study flowchart is shown in Figure 1. A total of 1451 patients underwent TAVI at 5 participating centres between January 2009 and August 2017. The follow-up ended on August 30, 2020. Among 1403 patients fulfilling the inclusion criteria, 100 patients were selected for the final analysis: 25 patients treated with chronic immunosuppression (15 patients received glucocorticoids, 4 received only non-steroid immunosuppression and 6 both glucocorticoids and non-steroids) and 75 patients not receiving immunosuppression. Baseline and procedural characteristics are shown in Table I. Details of immunosuppressive therapy and details of immunosuppression indications are shown in Tables II and III, respectively. After adjustment with optimal score matching, there were no baseline differences between the groups, except for a slightly higher EuroSCORE II risk score in the study group (p = 0.047) (Table I).
Table I
Baseline and procedural characteristics
| Variable | Non-IM (n = 75) | IM (n = 25) | P-value |
|---|---|---|---|
| Patients’ characteristics: | |||
| Age [years], median (IQR) | 82 (77.13–84.00) | 78 (74.00–81.89) | 0.4 |
| Gender (male), n (%) | 40 (53) | 15 (60) | 0.645 |
| EuroSCORE II (%), median (IQR) | 4.91 (3.15–8.49) | 3.91 (2.54–5.64) | 0.047 |
| Ejection fraction (%), median (IQR) | 55 (40–60) | 50 (40–60) | 0.57 |
| Haemoglobin [g/dl], median (IQR) | 11.70 (8.95–12.86) | 12 (8.80–13.71) | 0.63 |
| Estimated GFR [ml/min/1.73 m2] median (IQR) | 52.30 (41.5–60) | 54 (43–60) | 0.646 |
| Peripheral artery disease, n (%) | 15 (20) | 5 (20) | 1.000 |
| Procedures’ characteristics: | |||
| Transfemoral access, n (%) | 67 (89) | 22 (88) | 1.000 |
| Transapical access, n (%) | 4 (5) | 2 (8) | 0.638 |
| Other access, n (%) | 4 (5) | 1 (4) | 1.000 |
| Valve size, median (IQR) | 26 (26-29) | 27 (26-29) | 0.82 |
| Balloon-expandable valve1, n (%) | 21 (28) | 10(40) | 0.319 |
| Self-expandable valve2, n (%) | 54 (72) | 15 (60) | 0.319 |
| Procedural complications: | |||
| Life-threatening or disabling bleeding*, n (%) | 10 (13.33) | 5 (20) | 0.518 |
| Major vascular complication*, n (%) | 4 (5.33) | 1 (4) | 1.000 |
| Stroke, n (%) | 2 (2.67) | 1 (4) | 1.000 |
| New pacemaker, n (%) | 11 (14.7) | 5 (20) | 0.538 |
Table II
Details of immunosuppressive therapy in the study group (n = 25)
| Drug or drug combination | Number (%) |
|---|---|
| Prednisone | 7 (28) |
| Methotrexate and methylprednisolone | 3 (12) |
| Methylprednisolone | 2 (8) |
| Hydrocortisone | 1 (4) |
| Imatinib | 1 (4) |
| Cyclophosphamide | 1 (4) |
| Lack of data | 10 (40) |
Table III
Details of indications for immunosuppression in the study group (n = 25)
Procedural characteristics
All patients included in the analysis completed the follow-up at hospital discharge. There were no procedural differences between groups (Table I). The median prosthesis size was 27 mm in the study group and 26 mm in the control group (p = 0.820). No differences were found in the use of self-expanding and balloon-expandable valves between groups (p = 0.319).
In-hospital outcomes
The incidence of major vascular complications (4.0% vs. 5.3%) and the composite endpoint (40.0% vs. 20.0 %) were comparable between the groups (p = 1.000, p = 0.218, respectively). Individual composites of the endpoints were similar in the immunosuppression group and no immunosuppression group including the rate of procedural life-threatening or disabling bleeding (20.0% vs. 13.3%, p = 0.518), stroke (4% vs. 2%, p = 1.000) and new pacemaker implantation (20.0% vs. 14.7%, p = 0.538).
Long-term survival
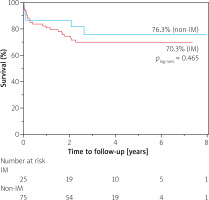
The median follow-up time was 2.7 years (IQR 1.8–3.9) in the control group and 2.7 years (IQR 1.9–4.7) in the study group (p = 0.33). The longest follow-up time was 7.4 years and 8.7 years in control and study groups, respectively.
At the end of the follow-up period, the survival rate was 70.3% in immunosuppression-treated patients and 76.3% in non-immunosuppression-treated patients. There were no significant differences in all-cause mortality between the immunosuppression-treated patients and patients without such treatment (p = 0.465; HR = 0.73; 95% CI: 0.30–1.77; Figure 2).
Discussion
This is the first study presenting data regarding the safety of TAVI in immunosuppression-treated patients over a long-term follow-up to 7–8 years. The main finding of our study is that the mortality and complication rates are comparable in immunosuppressed and non-immunosuppressed patients. Although there was a trend towards higher complication rates in patients treated with immunosuppression, the differences regarding major vascular complications, severe bleeding, and the need for permanent pacemaker implantation or stroke were comparable between the groups.
Data regarding patients with chronic immunosuppressive treatment who have undergone TAVI are scarce and inconclusive. In accordance with our results, another retrospective cohort study including 20 patients with previous immunosuppression (both steroids and non-steroids) and 262 patients without it showed that the risk of vascular access site complications and mid-term major adverse cardiovascular and cerebrovascular event was comparable following TAVI in the two groups [13]. Another retrospective analysis with a 763-day follow-up compared 25 patients receiving steroid treatment at the time of TAVI with 19 patients without such treatment [14]. The authors found that steroid-treated patients had higher incidence of minor vascular complications (44% vs. 23%, p = 0.024), femoral artery stenosis (16% vs. 5%, p = 0.036), and occlusion (8% vs. 1%, p = 0.014) and more frequently needed percutaneous intervention on the femoral artery, compared to non-steroid treated patients (32% vs. 15%, p = 0.031). However, no significant differences were found regarding more severe complications. On multivariate analysis, steroid treatment was the only predictor of minor vascular complications (RR = 2.65, 95% CI: 1.04–6.8, p = 0.042).
The reason for the encouraging outcomes following TAVI despite the frailty associated with immunosuppression might be related to three factors: (i) appropriate selection of TAVI candidates by Heart Teams, (ii) improved safety of new generation devices and (iii) increasing operators’ experience. However, there are also studies showing contradictory results. In a prospective study comparing TAVI outcomes between 48 patients on chronic glucocorticoid therapy and 1251 patients without such treatment during 1-year follow-up, more complications and higher 1-year mortality were observed in the immunosuppressed patients [15]. When analysing the Kaplan-Meier curves in our study, there is also an initial trend showing higher survival in the non-immunosuppressed group, but this difference is not significant in the long-term observation (Figure 2). The discrepancies in the incidence of severe complications between the studies are harder to explain, especially since our initial hypothesis assumed a higher percentage of adverse events in immunosuppression-treated patients. We speculate that the fact that our study group consisted of both steroid-treated patients and patients with other forms of immunosuppression may be partially responsible for that effect. It is plausible that different types of immunosuppressive therapy have various impacts on post-TAVI complication rates.
The state of the art mandates the Heart Teams to select AS patients for appropriate treatment [4, 16, 17]. In this demanding decision-making process, it is vital for experts to take into consideration all potentially important clinical information affecting potential therapy outcomes [18]. Although both steroidal and non-steroidal immunosuppressive therapies are important factors in TAVI planning, our study suggests that it should not be a reason to deprive patients of the benefits of TAVI. Another interesting concept comes from the use of multiparametric risk scores such as the InterMountain Risk Score (IMRS) [19, 20]. Classic TAVI risk scores include the Society of Thoracic Surgeons score (STS) and the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE I and II), which were developed to predict outcomes after surgical procedures, not percutaneous. Multiparametric risk scores tailored for TAVI could become an effective tool in procedure planning. With expanding development of percutaneous treatment technologies, TAVI will likely become an acceptable treatment option in the case of many comorbidities which were initially considered a contraindication to TAVI. For example, previously we found that patients with a bicuspid aortic valve, initially considered a contraindication to TAVI, also had comparable mortality to patients with a tricuspid aortic valve up to 10 years after the procedure, although the rate of neurological complications was higher in patients with a bicuspid aortic valve [21, 22]. This study showed that chronic immunosuppression should also not be a contraindication to TAVI. Further research is warranted to identify other high-risk patients who might benefit from TAVI, such as patients with low left ventricle ejection fraction, frailty or very elderly patients.
Our study has several limitations. First, the small sample size might have influenced the statistical significance of our analyses; hence independent verification of outcomes reported here should be performed in a larger study. Second, no information about dosing or length of the immunosuppression therapy was available, yet it is crucial for the magnitude of side effects [23]. Third, data regarding the indications for immunosuppressive therapy were missing in some patients. Therefore, we cannot distinguish between the effects of immunosuppression therapy itself and the underlying disease on TAVI outcomes [24]. Fourth, data regarding the length of immunosuppressive therapy after TAVI and its association with the long-term valve performance, as well as data regarding the specific causes of death, were unavailable. Finally, our analysis did not include an additional control group of patients treated with surgical aortic valve replacement.
Conclusions
Our study showed comparable safety of TAVI and comparable long-term mortality after TAVI in patients with or without previous chronic immunosuppressive therapy over a median of 2.7 years, adding to the previously available evidence showing that TAVI might be a viable treatment option in such patients. Regarding the limitations of our analysis and paucity of literature data, more research is required to provide definite answer regarding the efficacy and safety of TAVI in this fragile population of patients.