Introduction
Subclavian artery aneurysms (SAAs) are very rare, representing about 1% of peripheral arterial aneurysms [1]. The underlying etiologies of SAA formation include atherosclerosis, thoracic outlet syndrome, collagen disorders, infection [2] and iatrogenic or traumatic injuries [3]. Most true SAAs are caused by atherosclerosis and thoracic outlet syndrome, whereas trauma is a more common etiology for pseudoaneurysms of the subclavian artery.
Mycotic SAAs are a very rare disorder [4]. A mycotic aneurysm is an infection of the vessel wall, which can be bacterial, fungal, or viral in origin [5]. It can affect any arteries of the body and it may cause fetal consequences, such as aneurysmal expansion, aneurysmal rupture, infection relapse and even deterioration, etc. [6]. It poses challenges with regard to the diagnosis and treatment of choice. In the literature, information on clinical features, diagnosis, management and outcomes of this disorder is limited.
Aim
This article aims to give an overview of current knowledge on clinical features, diagnostic and management strategies, and outcome evaluations of mycotic SAAs.
Material and methods
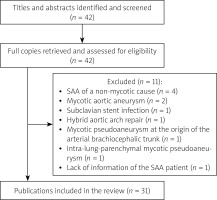
Comprehensive literature was retrieved from the PubMed, Google Scholar and “Baidu” Scholar for articles published from 2000 to 2023. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement guidelines were followed in this review. The used search terms included “subclavian artery”, “aberrant subclavian artery”, “lusorian artery”, “arteria lusoria”, “subclavian artery aneurysms”, “endovascular interventional treatment”, “stent graft”, “coil embolization”, “hybrid”, “surgical operation”, “hemoptysis”, and “mycotic”, etc., in the literature search. The inclusion criteria were prospective or retrospective studies, case series and case reports. The primary exclusion criteria were publications: SAA of a non-mycotic cause (n = 4), mycotic aortic aneurysm (n = 2), subclavian stent infection (n = 1), hybrid aortic arch repair (n = 1), mycotic pseudoaneurysm at the origin of the arterial brachiocephalic trunk (n = 1), intra-lung-parenchymal mycotic pseudoaneurysm (n = 1), and lack of information of the SAA patient (n = 1). As a result, a total of 31 articles were included and 11 articles were excluded. The inclusion and exclusion of literature retrieval are shown in Figure 1.
Statistical analysis
IBM SPSS statistics version 26.0 software was applied for statistical analysis. The measurement data were expressed as mean ± standard deviation and were compared by the independent t test, while the categorical data were expressed as numbers and percentages. Logistic regression analysis was conducted to determine the predictive factors of the outcomes of patients with an SAA. P < 0.05 was considered of statistical significance.
Results
A total of 31 articles [7–37] were included, with 25 (80.6%) case reports and 6 (19.4%) small case series [13, 22, 24, 25, 31, 35]. Unfortunately, no retrospective or prospective research articles were available from the literature.
There were in total 33 patients: 32 patients had a single SAA, and 1 patient had multiple SAAs but the number of aneurysms of this patient was not given; however, only one aneurysm of her multiple SAAs required interventional therapy [25]. Moreover, 2 patients with a single SAA developed a recurrent aneurysm after the initial interventional therapy [30, 33]. Thus, in total 35 SAAs of 33 patients were analyzed.
Patients were at the age of 49.7 ±23.3 (range: 6–89; median: 58) years (n = 33). There were 18 (54.5%) male and 15 (45.5%) female patients. The etiologies of SAAs were unknown in 4 patients [7, 15, 30, 33]. In the remaining 29 patients, non-abscess infections and abscesses were the principle causes leading to the formation of SAAs (Table I).
Table I
Etiology of mycotic subclavian artery aneurysms
| Etiology | n (%) |
|---|---|
| Non-abscess infection: | 22 (75.9) |
| Pulmonary infection: | 6 (27.3) |
| Pulmonary aspergillosis [9, 27, 34, 32] | 4 (66.7) |
| Lung infection [12] | 1 (16.7) |
| Mycobacterium tuberculosis [36] | 1 (16.7) |
| Local infection [22]: | 5 (22.7) |
| Infection of the subclavian artery stent and axillofemoral graft [22] | 1 (20) |
| Nasal cellulitis [10] | 1 (20) |
| Pyrogenic spondylitis [26] | 1 (20) |
| Syphilitic arteritis [21] | 1 (20) |
| Wound infection [25] | 1 (20) |
| Systemic infection: | 4 (18.2) |
| Bloodstream and thoracic infections [31] | 1 (25) |
| Endocarditis [18] | 1 (25) |
| Sepsis [11] | 1 (25) |
| Septicemia [24] | 1 (25) |
| Local and systemic infections: | 1 (4.5) |
| Clavicular infection and bacteremia [17] | 1 (100) |
| Local, lung and systemic infections: | 1 (4.5) |
| Methicillin-resistant Staphylococcus aureus (MRSA) pneumonia, mediastinitis and sepsis [23] | 1 (100) |
| Other possible infection: | 5 (22.7) |
| Intravenous drug use [14, 20, 35, 35] | 4 (80) |
| Intra-arterial drug injection [13] | 1 (20) |
| Abscess: | 5 (17.2) |
| Local abscess: | 4 (80) |
| Sternum and left rib[19] | 1 (25) |
| Back [29] | 1 (25) |
| Mediastinal and sternoclavicular joint abscess [8] | 1 (25) |
| Paravertebral and mediastinal [16] | 1 (25) |
| Pulmonary abscess: | 1 (20) |
| Pulmonary abscess [25] | 1 (100) |
| Abscess and non-abscess infection: | 2 (6.9) |
| Pulmonary aspergillosis + liver and splenic abscesses [37] | 1 (50) |
| Retrosternal abscess + left sternoclavicular septic arthritis [28] | 1 (50) |
Detection of pathogenic microorganisms was not mentioned for 5 patients in 5 reports [13, 14, 18–20]. In the remaining 28 patients, the cultures of blood and (or) sputum were negative in 2 patients [7, 36], and positive results of microbiology were obtained in 26 patients by 30 detections with 15 kinds of microorganisms detected in 25 patients and polymicrobial growth was found in 1 patient. The most common microorganisms were Aspergillus and methicillin-sensitive Staphylococcus aureus. The investigations included 29 (29/37, 78.4%) cultures and 2 (2/37, 5.4%) microbiological analyses. Meanwhile, investigative methods for the microorganisms were unknown for 6 (6/37, 16.2%) detections of 6 patients. In general, 37 specimens were sent for investigations of the microorganisms. The specimens for cultures were blood (n = 11), wound tissues (n = 6), surgical specimens (n = 3), pus (n = 3), sputum (n = 2), pleural effusion (n = 1), Hickman line tip (n = 1), urine (n = 1) and an unknown specimen (n = 1). Microbiological analysis was done for 2 patients with pus in 1 patient and a surgical specimen in another. In the 6 patients in whom the detection methods were not reported, the specimens for investigations were bronchial wash and brushings in 1 patient whereas the specimens were not mentioned for the other 5 patients (Table II).
Table II
Microorganisms causing mycotic subclavian artery aneurysms
| Microorganism | n (%) | Culture (n) | Microbiological analysis (n) | Detection method unknown (n) |
|---|---|---|---|---|
| Aspergillus[9, 27, 31, 32, 34, 37] | 6 | Pleural effusion (1) [31] | Bronchial wash and brushings (1) [27]Unknown (4) [9, 32, 34, 37] | |
| Methicillin sensitive staphylococcus aureus [8, 11, 17, 22, 28, 30] | 6 | Blood (4) [11, 17, 22, 28]Hickman line tip (1) [11]Pus (1) [22]Surgical specimen (1) [30]Unknown (1) [8]Wound tissue (1) [28] | Surgical specimen (1) [17] | |
| Escherichia coli [16, 24] | 2 | Blood (1) [24]Urine (1) [24] | Pus (1) [16] | |
| Methicillin-resistant staphylococcus aureus [23, 25] | 2 | Blood (1) [23]Wound tissue (1) [25] | ||
| Staphylococcus aureus [35] | 2 | Blood (1) [35]Wound tissue (2) [35] | ||
| Streptococcus viridans [15, 35] | 2 | Blood (1) [15]Wound tissue (1) [35] | ||
| Streptococcus pneumoniae [10] | 2 | Pus (1) [29]Surgical specimens (1) [10] | ||
| Burkholderia pseudomallei [33] | 1 | Blood (1) [33]Surgical specimen (1) [33] | ||
| Enterobacter cloacae [26] | 1 | Blood (1) [26] | ||
| Polymicrobial growth [31] | 1 | Blood (1) [31] | ||
| Salmonella group B [12] | 1 | Sputum (1) [12] | ||
| Veillonella [35] | 1 | Wound tissue (1) [35] | ||
| Prevotella species [29] | 1 | Pus (1) [29] | ||
| Syphilis [21] | 1 | Unknown (1) [21] | ||
| Xanthomonas maltophilia [25] | 1 | Sputum (1) [25] |
There were in total 73 clinical symptoms in all 33 patients including 69 (94.5%) symptoms for patients with a primary SAA and 4 (5.5%) symptoms for the 2 patients at the time of aneurysmal recurrence. Fever was the most common symptom (n = 20, 27.4%), followed by a pulsatile/painful mass (n = 10, 13.7%), local pains (in the arm, hand, neck, back, shoulder, chest or periscapular region) (n = 10, 13.7%) and hemoptysis (n = 9, 12.3%). In the 9 patients with hemoptysis, the causes of hemoptysis were lung infection in 5 (55.6%) patients (pulmonary abscess [25], pulmonary erosive fungal infection [32], pneumonia of the right middle lobe [35], and pulmonary aspergillosis [27, 37], aneurysmal rupture in 3 (33.3%) patients [12, 20, 34], and acute expansion of the pseudoaneurysm in 1 (11.1%) patient [26]. Horner’s syndrome was present in 1 patient [20].
Initial antimicrobial therapy was mentioned for 18 patients [7–9, 12, 16, 18, 19, 21, 23, 24, 26, 29, 31–33, 35, 37]. The antibacterial drugs were antibacterial agents (cefotaxime, ceftazidime, cephalexin, meropenem, or vancomycin) for 14 (77.8%) patients, both antibacterial and antifungal agents (amphotericin B) for 3 (16.7%) patients and only an antifungal agent for 1 (5.6%) patient. Postoperative antibacterial drug use was reported for 18 patients [10–12, 14, 16, 17, 22–24, 26–29, 33, 35, 37] with a duration of 7.5±5.1 (range: 2–20; median: 6) months (n = 13).
The white blood cell count was mentioned for 16 patients [7, 9, 10, 14, 15, 18, 19, 23–29, 33, 37]. In 3 patients, it was described as “increased” or “normal”, and in the remaining 13 patients, a concrete value was given. A normal white blood cell count was noted in 4 (25%) patients [9, 15, 18, 29], leukopenia was present in 2 (12.5%) patients [24, 37], and leukocytosis was present in 10 (62.5%) patients [7, 10, 14, 19, 23, 25–28, 33]. The mean white blood cell count was 11.2 ±6.3 (range: 0.8–21.5; median: 12.8) × 109/l (n = 13). C-reactive protein (CRP) was mentioned for 9 patients [7, 18, 19, 23, 24, 26, 29, 32, 36]. Normal CRP was noted in 3 (33.3%) patients [18, 19, 29], and elevated CRP was observed in 6 (66.7%) patients [7, 23, 24, 26, 32, 36]. The mean CRP was 24.0 ±23.9 (range: 5.4–70; median: 19.0) mg/dl (n = 5).
The aneurysmal dimension was reported for 22 aneurysms of 21 patients with a mean of 5.1 ±4.2 (range: 1.4–21; median: 3.8) cm (n = 22) [7, 8, 10–12, 16–19, 23, 25, 26, 28–30, 33–35, 37].
A total of 52 diagnostic methods for SAA were used for 34 aneurysms of 32 patients, including, computed tomography (n = 18, 34.6%) [7–11, 15, 19, 20, 24, 26, 28, 29, 31–33, 35, 37], computed tomographic angiogram (n = 15, 28.8%) [9–12, 14, 16, 17, 19, 22, 23, 28, 30, 34, 36], angiogram/arteriogram (n = 11, 21.1%) [8, 18, 19, 21, 25, 27, 32, 33, 35], echocardiography/ultrasonography (n = 5, 9.6%) [15, 18, 21, 35], magnetic resonance imaging (n = 2, 3.8%) [7, 18], and magnetic resonance angiogram (n = 1, 1.9%) [21].
The aneurysmal formation time (the time from the impact of the etiology to its formation) was reported for 19 patients with a mean of 41 ±10.8 (range: 0.3–48; median: 1.2) months [8–10, 12, 14, 15, 19, 20, 24, 25, 28–30, 32–35, 37]. The formation time was much longer in intravenous drug users than in patients with an abscess (intravenous drug user: 16.2 ±27.6 months vs. abscess: 1.3 ±0.6 months, p = 0.002) or lung infection (intravenous drug user: 16.2 ±27.6 months vs. lung infection: 1.9 ±1.4 months, p = 0.002).
The dimension of the SAA was reported for 22 aneurysms of 22 patients with a mean of 5.1 ±4.2 (range: 1.4–21; median: 3.5) cm [7, 8, 10–12, 16–19, 23, 25, 26, 28–30, 33–35, 37]. The location of the SAA was not mentioned for 1 patients. In the remaining 32 patients, the aneurysms were derived from the left subclavian artery in 14 (43.8%) patients [9, 10, 12, 15, 19, 21, 24, 25, 28, 29, 32, 36, 37], and from the right in 18 (56.3%) patients [7, 8, 11, 14, 16–18, 20, 22, 23, 26, 27, 30, 31, 33–35] (one of which was from the right aberrant subclavian artery [7]). In the case of two aneurysms, their extra- or intrathoracic positions were not mentioned. As regards the remaining 33 aneurysms, 12 (36.4%) were extrathoracic [7, 10, 14, 18, 21, 23, 25, 30, 31, 35, 36] and 21 (63.6%) (including 1 recurrent one) were intrathoracic [8, 9, 11, 12, 15–17, 19, 20, 22, 24–29, 32, 33, 35, 37]. 33 aneurysms (including 31 primary and 2 recurrent) were pseudoaneurysms [8–16, 18–37], whereas 2 were true aneurysms [7, 17]. In 5 (15.2%) patients, the aneurysms were ruptured [12, 14, 20, 30, 34].
Local abscess formation was observed in 7 (21.2%) patients [10, 14, 16, 19, 22, 24, 28], aneurysmal thrombus in 7 (21.2%) patients [8, 9, 11, 16, 18, 24, 31], and aneurysmal expansion in 9 (27.3%) patients [9, 11, 23, 25, 26, 28, 30, 33, 36].
Associated aneurysms of other arteries/aorta were found in 4 (12.1%) patients: they were descending aorta and popliteal aneurysms [8], descending aorta aneurysm [24], descending thoracic and thoracoabdominal aortic aneurysm [29] and coronary artery aneurysms [33].
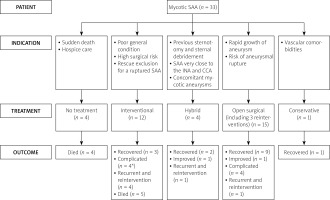
The interventional indications were described for 9 patients: poor general condition [23, 25], high surgical risk [12, 25], rescue exclusion for a ruptured pseudoaneurysm [14, 34], aneurysmal rupture tendency [18, 26], aneurysm expansion [23, 26], wound infection [25], poor surgical access to the lesion [12] and need of chemotherapy with no time of recovery from open surgery [32]. The indications for hybrid procedures were previous sternotomy and sternal debridement [8], SAA originating very close to the innominate and common carotid arteries [11], concomitant mycotic aneurysms including a rupture [29] and requiring innominate artery ligation and extra-anatomic bypass in redo operation [30]. The surgical indications for open surgery were rapid growth of the aneurysm and substantial risk of aneurysmal rupture [28, 33]. The indication for conservative treatment was described as “due to the vascular co-morbidities” [24].
Overall outcome estimation revealed that 14 (42.4%) patients recovered, 1 (3.0%) improved, 9 (27.3%) experienced complications (2 required reinterventions and 1 died later), 4 (12.1%) had recurrence after the initial interventions (1 failed interventional case was classified as recurrent) (4 required an reintervention and 1 of them died later), 6 (18.2%) had reinterventions and 9 (27.3%) patients died. The 9 deaths included 4 pre-treatment deaths and 5 post-treatment deaths. The 4 pre-treatment deaths were due to rupture of the pseudoaneurysm in 3 and after long-term hospice care in 1 patient, and the 5 post-treatment deaths were unrelated to the surgical or interventional procedures (massive pulmonary bleeding, brain edema and relapse of leukemia in 1 patient each and unknown causes in 2 patients).
No treatment was given to 4 patients due to preoperative sudden death in 3 patients [9, 15, 20] and hospice care in 1 patient who also died later [31]. In the remaining 29 patients, interventional, hybrid, open surgical procedures and conservative treatment were performed in 12 (41.4%), 4 (13.8%), 15 (51.7%) (including 3 reinterventions) and 1 (3.4%) patient. An urgent intervention/operation was needed in 6 (6/31, 19.4%) procedures [26, 30, 33–35]. Patients were on a follow-up of 15.8 ±19 (range: 1.8–84; median, 10) months (n = 22). Mortality only occurred in the interventional group with a mortality rate of 41.7%. The causes of death were brain edema, pulmonary bleeding and relapse of leukemia in 1 patient each and unknown in 2 patients. Endoleak occurred in 2 (2/12, 16.7%) patients in the interventional and in 1 (1/4, 25%) patient in the hybrid group. The recovery rates of 4 groups were 25%, 50%, 60% and 100%, respectively (Table III). The outcomes of the treatment of cases are shown in Figure 2.
Table III
Outcomes of patients receiving different treatments
| Management | Procedure | n (%) | Outcome (R/I/C/R&R/D), n (%) |
|---|---|---|---|
| Interventional: | 12 (41.4) | 3 (25)/0 (0)/4 (33.3)*/2 (16.7)/5 (41.7) | |
| Stenting | 5 (41.7) | 0 (0)/0 (0)/2 (40)/0 (0)/3 (60) | |
| Coils | 5 (41.7) | 2 (40)/0 (0)/1 (20)*/2 (40)**/2 (40) | |
| Stenting + coils | 2 (16.7) | 1 (50)/0 (0)/1 (50)/0 (0)/0 (0) | |
| Hybrid | Extra-anatomical bypass + stenting × 3, right brachial cutdown, stenting and dilation of the right subclavian artery × 1 | 4 (13.8) | 2 (50)/1 (25)/0 (0)/1 (25)/0 (0) |
| Surgical: | 15 (51.7)*** | 9 (60)/1 (6.7)/4 (26.7)/1 (6.7)/0 (0) | |
| Resection | 8 (53.3) | 5 (62.5)/0 (0)/2 (25)/1 (12.5)/0 (0) | |
| Ligation | 4 (26.7) | 3 (75)/0 (0)/1 (25)/0 (0)/0 (0) | |
| Plication | 1 (6.7) | 0 (0)/1 (100)/0 (0)/0 (0)/0 (0) | |
| Bilateral subclavian transposition | 1 (6.7) | 1 (100)/0 (0)/0 (0)/0 (0)/0 (0) | |
| Explantation of infected stent | 1 (6.7) | 0 (0)/0 (0)/1 (100)/0 (0)/0 (0) | |
| Conservative | 1 (3.4) | 1 (100)/0 (0)/0 (0)/0 (0)/0 (0) |
Figure 2
Diagram showing outcomes of treatment of cases
*One patient was complicated and died later, CCA – common carotid artery, INA – innominate artery, SAA – subclavian artery aneurysm.
Logistic regression analysis revealed that none of the following variables were significant predictive factors of the outcomes of patients with an SAA: gender (p = 0.86), age (p = 0.16), etiology (p = 0.89), fever (p = 0.72), hemoptysis (p = 0.69), laterality of SAA (p = 0.74), true or false aneurysm (p = 0.49), aneurysmal rupture (p = 0.36), intra- or extrathoracic location (p = 0.42), abscess formation (p = 0.74), a thrombosed aneurysm (p = 0.76), aneurysm expansion (p = 0.56), and treatment of choice (p = 0.41).
Discussion
The contaminated arterial injuries and the arterial wall erosion from an existing abscess formation are the two main mechanisms of SAA formation [14]. Moreover, both primary and secondary immunodeficiencies can be risk factors leading to the development of mycotic SAAs. Primary immunodeficiency diseases are a group of genetic disorders affecting distinct components of the innate and adaptive immune system [38]. The primary immunodeficiency diseases were described as “well-defined syndromes with immunodeficiency” or “combined immunodeficiencies” by Al-Herz et al. [39] in their classification article. However, patients with a primary immunodeficiency were not found in the present patient cohort. Secondary immunodeficiencies include infectious diseases (including HIV), environmental stress, age extremes (prematurity and old age), surgery and trauma, immunosuppressive drugs, and genetic and metabolic disease [40]. All patients of this patient cohort had a history of common infection. In addition, 1 patient had HIV infection [20]. To put aside the infection and history of surgery, but to focus on the drug-induced secondary immunodeficiencies while talking about the risk factors responsible for the development of the SAAs, we noted that 8 (24.2%, 8/33) cases of drug-induced secondary immunodeficiencies including 6 (75%, 6/8) cases of chemotherapy (for acute leukemias (n = 4) [9, 25, 32, 37], for diffuse large B cell lymphoma (n = 1) [31], or for carcinoma of the uterine cervix with liver and bone metastasis (n = 1) [25]), and 2 (25%, 2/8) cases of anti-rejection therapy (for kidney transplantation (n = 1) [16] and for allogenic peripheral blood stem cell transplantation (n = 1) [34]).
Patients with an SAA can be asymptomatic and found by incidental work-ups. In symptomatic patients, pains in the chest or shoulder, and a painful pulsatile mass in the supraclavicular region are the common symptoms. Secondary complications, such as rupture, thrombosis, or embolization, are rare events. Aneurysms of other arteries were reported to be found in 33–47% of patients with an SAA at the time of diagnosis [41]. In comparison, in the present patient cohort, associated aneurysms of other arteries/aorta were found in 12.1% of patients with a mycotic SAA.
Vierhout et al. [2] classified the symptoms of SAA patients into 5 groups: local signs (pulsating mass, shoulder or chest pains), rupture (hemoptysis, hemothorax, open rupture and hemorrhage), compression (dysphagia, dyspnea, brachial plexopathy, Horner’s syndrome and hoarseness), thrombosis (ischemic arm) and embolization (numb or cyanotic fingers, and cerebral infarction). This cohort of patients exhibited all the above-mentioned symptoms except for dyspnea, hemothorax and brachial plexopathy. Moreover, cough [9, 17], weight loss [15, 25, 27, 36] and mental status alteration [23, 28, 33] could be alternative symptoms of patients with a mycotic SAA.
Diagnoses of mycotic SAAs can be established based on microorganism isolations from the human fluids such as blood, urine and sputum, as well as cultures of the infected tissues. However, some cultures may show negative results due to early empirical therapy. Chest X-ray could show a mass lesion in the left retroclavicular region even though it would not lead to a definite diagnosis [12]. Other medical imaging methods including ultrasound, angiogram, computed tomography and magnetic resonance imaging are helpful in demonstrating the configuration of the aneurysm of the left subclavian artery and in judgement of the possible mechanisms of aneurysmal formation [32].
The treatment of SAAs is usually surgical due to the possibility of rupture, embolism, or thrombosis if untreated. A high mortality rate due to rupture, irrespective of the aneurysmal size, has been reported. Aneurysms in the second or third portion of the artery may be accessed through a supraclavicular incision. However, intrathoracic aneurysms usually require a medial sternotomy. Davidović et al. [13] reported a series of 14 patients with SAA treated with a supraclavicular or transsternal approach, depending on the locations of the aneurysms: a supraclavicular approach in 12 and a combined transsternal and supraclavicular approach in 2 patients. A median sternotomy was the preferred approach for right-sided intrathoracic SAAs, while a left thoracotomy is suitable for left-sided intrathoracic SAAs [13]. When the SAA is proximal and involves the vertebral artery, a transsternal approach is more convenient [42]. Stent graft deployment into the subclavian artery would not be practical if the SAA is at the bifurcation of the innominate artery, and an extra-anatomic bypass would be required if the right vertebral artery originated from the aneurysm. An open surgical approach to access an aneurysm at the bifurcation of the innominate artery would require a partial transsternal approach [42]. Cardiopulmonary bypass was once used for the resection of a subclavian artery pseudoaneurysm [33]. Open surgical repair of SAA was often associated with increased mortality and morbidity despite continuous improvement of surgical techniques [43]. The postoperative complication rate could be high up to 46% as reported by Salo et al. [44] in a series of 13 patients with open surgery for SAAs.
The difficulty of the surgical approach and of the surgical field exposure, intraoperative trauma and significant complications associated with open surgery make people recognize the advantages and necessity of endovascular interventional treatment [45]. A subclavian stent graft for a subclavian artery injury was first reported in 1991 [46]. The endovascular treatment of subclavian artery lesions is less invasive than open surgical repair with a low rate of complications [47]. However, endovascular exclusion of the aneurysm with a stent was not possible in true aneurysms as the aneurysm lacks a neck for the deployment of a stent graft [48]. In extremely friable and thin-walled SAAs, a stent graft is often impractical. Thus, a hybrid approach becomes necessary in this situation. Artery continuity can be reestablished by interposition grafting, primary reanastomosis, or extra-anatomic bypass procedures. Conservative management was described for patients who were contraindicated for an interventional or surgical procedure, but patients carry higher risks of aneurysmal rupture [2].
The lack of data was the primary drawback of this study. As an extremely rare disease, the small sample size of patients resulted in a lack of accuracy in statistical analysis. Due to the lack of data and small samples, the t-test to evaluate the aneurysmal formation time caused by different pathogenic factors was not accurate enough, and the recovery rate of conservative management was the highest among the four treatment options. The expected count of some cells was less than 5, making the χ2 test to evaluate the effectiveness of treatment strategies fruitless. These results have to be reevaluated in the future on the basis of more abundant patient information.
Conclusions
Mycotic SAAs are rare but can be sometimes fatal. Once a diagnosis of mycotic SAA is made, sensitive antibacterial drugs are applied immediately to control the infection and control aneurysmal progression. Early treatment is conducted as soon as possible to avoid aneurysmal rupture. A decision of treatment of choice is made based on the patient’s specific situation. Antibacterial drug use is continued for about 6 weeks after surgical or interventional therapy.