Introduction
Apoptosis, one of the forms of programmed cell death, is fundamental for homeostasis of the skin. Physiologically, it enables the development and maintenance of normal thickness and structure of the epidermis. It also provides an important defence mechanism against neoplastic transformation that may be induced in the skin by various harmful stimuli [1, 2]. Unlike keratinocytes and other cell types residing in the epidermis, melanin-producing melanocytes are considered quite resistant to apoptosis [3]. In vitro studies show that normal melanocytes can enter the apoptotic pathway when exposed to dopamine [4], homocysteine [5], interferon-γ (INF-γ) [6], cytosolic poly (dA:dT) (an analogue of viral non-CpG ds DNA) [7], the synthetic tyrosine analogue 4-TBP (4-tert-butyl-phenol) [8], particulate matter 2.5 (PM 2.5) [9], and ultraviolet (UV) radiation [3]. Exposure of the skin to UVA and UVB radiation is a well-described factor that increases nitric oxide (NO) levels in the epidermis via activation of inducible nitric oxide synthase (iNOS) in keratinocytes, Langerhans cells and melanocytes [10].
As a messenger molecule that affects many various cell signalling pathways, NO is known to be involved in numerous physiological and pathological processes in target tissues, including vascular homeostasis or inflammatory and immune responses [11]. A large body of in vitro evidence demonstrates that NO is also a potent modulator of programmed cell death, with the ability to both induce and prevent apoptosis. The pro- or antiapoptotic effect appears to be cell-type specific, but it depends also on the NO concentration and its delivery method, exposure time, and possible interactions with other molecules present in the biological milieu, such as oxygen or superoxide anion, thiols or proteins, antioxidants, etc. [12, 13]. NO was reported to affect apoptosis in a wide variety of cells, including macrophages, neurons, pancreatic β-cells, thymocytes, chondrocytes, hepatocytes, endothelial cells, eosinophils, or B lymphocytes [13]. Very little is known about the possible modulatory effect of NO on programmed death of epidermal melanocytes, and these scarce data are mainly related to melanoma cells. For instance, overproduction of NO via induction of NOS was found to promote apoptosis in hypoxic murine melanoma models and human A375-S2 melanoma cells [14, 15]. On the other hand, it has been suggested that constitutive NO production prevents apoptosis and is therefore essential for the survival of human melanoma cells. Reduced levels of endogenous NO due to iNOS inhibition induced apoptosis in patient-derived melanoma cells, but interestingly not in normal human epidermal melanocytes [16]. Ivanova et al. [17, 18] described NO-induced detachment of human epidermal melanocytes from the extracellular matrix in vitro and suggested apoptotic cell death as a major part of the underlying mechanism. Interestingly, the cell response to NO was modified by the level of cell pigmentation. In light of recent studies [19, 20], the cell pigmentation phenotype appears to be an important factor modulating the biochemical behaviour of melanocytes in response to various intrinsic and extrinsic stimuli.
Aim
The present study aims to answer the question of whether NO is indeed able to induce apoptosis in normal human epidermal melanocytes and whether the pigmentation phenotype of the cells could be a factor modulating their response to NO in this respect.
Material and methods
Reagents and compounds
N-[4-[1-(3-Aminopropyl)-2-hydroxy-2-nitrosohydrazino]butyl]-1,3-propanediamine (spermine–nitric oxide complex; SPER/NO), Dulbecco’s Phosphate Buffered Saline (D-PBS), Hoechst 33342 fluorescent dye, and Trypan Blue solution (0.4%) were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Growth medium M-254 supplemented with Human Melanocyte Growth Supplement (HMGS), and antibiotic/antimycotic solution of gentamicin and amphotericin B were obtained from Cascade Biologics/GIBCO Life Technologies (Carlsbad, CA, USA).
Cell culture
Primary human epidermal melanocytes derived from the neonatal foreskin of lightly and darkly pigmented donors (HEMn-LP; Lot # 423339 and HEMn-DP; Lot # 765194) were cultured in the supplemented medium in a humidified atmosphere of 5% CO2 at 37°C. All experiments were performed using cells at passages 3-5. SPER/NO was dissolved directly in the culture medium immediately before use.
Analysis of cell morphology, proliferation, and viability
HEMn were seeded in complete medium and allowed to attach and grow for 48 h. Then, SPER/NO (50–500 μM) was added in a fresh medium, and the cells were incubated for 24 h. Morphological changes of HEMn after SPER/NO treatment were evaluated using a reversed-phase microscope. Cell proliferation was measured by 5-bromo-2′-deoxyuridine (BrdU) incorporation during DNA synthesis using Cell Proliferation ELISA BrdU assay (Roche, Basel, Switzerland) according to the manufacturer’s protocol. Determination of the number of viable cells was based on quantitation of ATP using the CellTiter-Glo®Luminescent Cell Viability Assay (Promega, Madison, WI, USA) according to the manufacturer’s protocol.
Detection of apoptosis by flow cytometry
Adherent melanocytes incubated with or without SPER/NO (100, 250, and 500 μM) for 24 h were harvested by trypsinization and pooled with the cells floating in the medium. The cells were double-stained with the CF488A-Annexin V and PI (propidium iodide) Apoptosis Assay Kit (Biotum, Fremont, CA, USA) following the manufacturer’s instructions, and then they were analysed by flow cytometry using a FACSAria II flow cytometer (BD, Franklin Lakes, NJ, USA). CF488A-Annexin V-positive/PI-negative cells were identified as early apoptotic, whereas CF488A-Annexin V-positive/PI-positive cells were classified as late apoptotic (secondary necrotic) cells.
Nuclear staining with Hoechst
The morphological changes in HEMn incubated with or without SPER/NO (50–500 μM) for 24 h were determined by DNA staining with Hoechst 33342 fluorescent dye and were visualized by AxioVert.A1FL inverted microscope with epi-fluorescence LED (Carl Zeiss Microscopy, NY, USA).
DNA fragmentation assay
HEMn were incubated with or without 500 μM SPER/NO for 24 h. Adherent and detached cells were then collected, and genomic DNA was extracted from the cells with the Apoptotic DNA Ladder Kit (Roche, Basel, Switzerland). The extracted DNA and the DNA ladder marker (Gene Ruler™ 1000 bp DNA Ladder, Thermo Scientific™) were loaded onto 1% agarose gel, and DNA fragments were separated by electrophoresis. The DNA ladder pattern was visualized on a UV transilluminator by SYBR®Green staining (Sigma-Aldrich, St. Louis, MO, USA). Images were obtained using a ChemiDOC™ XRS+Imaging System (Bio-Rad Laboratories, Hercules, CA, USA).
Caspase activity assay
Activities of caspases 3/7, 8, and 9 in cultured melanocytes incubated with or without SPER/NO (50–500 μM) for 24 h were measured using the Caspase-Glo®-3/7, -8, and -9 Assay kits (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The luminescence intensity (RLU), proportional to the activity of caspase, was read in a TRIAD LT microplate reader (Dynex Technologies, Chantilly, VA, USA).
Gene expression analysis
Expression of the genes encoding B cell lymphoma 2 (BCL-2) protein and BCL-2-associated X protein (BAX) was analysed by real-time reverse transcription polymerase chain reaction (RT-PCR) technique. Total RNA was extracted from the cultured cells using the Direct-zol™RNA MiniPrep Kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s protocol. RNA samples were treated with DNase I solution and additionally purified using the OneStep™PCR Inhibitor Removal Kit (Zymo Research, Irvine, CA, USA). First-strand cDNA synthesis was performed with 1 μg of total RNA using the iScript™Reverse Transcription Supermix (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions. Expression of mRNA for BAX, BCL-2, and GAPDH was determined using the SsoAdvanced™Universal SYBR®Green Supermix with CFX Connect Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). The reaction mixture contained 10 μl SsoAdvanced™Universal SYBR®Green Supermix (2×), 1 μl PrimePCR SYBR Green Assay (20x) for BAX (qHsaCED0037943), BCL-2 (qHsaCED0057245), and GAPDH (qHsaCED0038674), 0.5 μl template cDNA, and 8.5 μl nuclease-free water. The reaction conditions were as follows: denaturation at 95°C for 30 s, followed by 40 cycles at 95°C for 5 s and 60°C for 30 s. Each sample was run in triplicate, and threshold cycle (Ct) values were collected. BCL-2 and BAX mRNA levels were normalized to GAPDH. Fold change in expression of BCL-2 and BAX was calculated by the 2–ΔΔCt method.
Results
Nitric oxide induces cell morphological changes and reduces melanocyte proliferation and viability
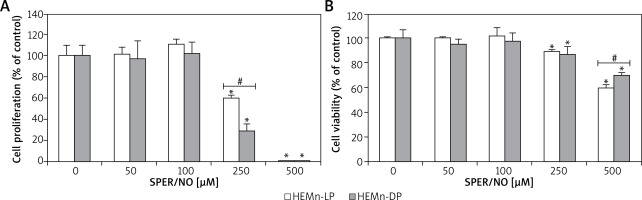
As shown in Figures 1 and 2, the exposure of melanocytes to NO released from SPER/NO that was added to the culture medium at relatively low concentrations (50 and 100 μM) had no effect on cell morphology, proliferation, and viability. However, the presence of 250 and 500 μM SPER/NO in the culture medium led to dendrite retraction, cell rounding, and cell detachment from the monolayer. In addition, a significant reduction or even complete inhibition of cell proliferation, as well as significant decrease in cell viability, were observed.
Figure 1
Morphological changes observed in HEMn treated with various concentrations of SPER/NO (50–500 μM) and cultured for 24 h under an inverted microscope (magnification: ×200). The arrows indicate detached cells
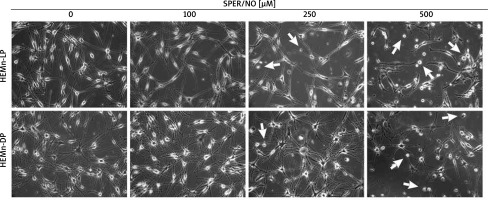
Figure 2
HEMn were treated with various concentrations of SPER/NO (50–500 μM) and cultured for 24 h. A – Cell proliferation was assessed using Cell Proliferation ELISA BrdU (Roche, Basel, Switzerland). B – Cell viability was determined by measuring intracellular ATP levels using CellTiter-Glo®Luminescent Cell Viability Assay (Promega, Madison, WI, USA). Values express as percentage of control, and represent the mean ± SEM of 3 independent experiments; *p < 0.05 versus untreated control (melanocytes cultured without SPER/NO), #p < 0.05 between HEMn-LP and HEMn-DP
Nitric oxide induces apoptosis in melanocytes
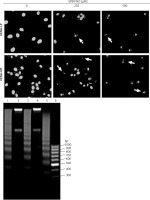
The results of Annexin V/PI flow cytometry (Figure 3) demonstrated significantly enhanced apoptosis only in HEMn exposed to the highest SPER/NO concentration used. HEMn-LP were more prone to NO-induced apoptosis than HEMn-DP. After the exposure to 500 μM SPER/NO, the total percentages of apoptotic LP and DP melanocytes were assessed as 95% and 18%, respectively. Hoechst 33342 staining showed that HEMn treatment with 250 and 500 μM SPER/NO induced cell shrinkage, chromatin condensation, and nucleus fragmentation (Figure 4, upper panel). At 500 μM SPER/NO, DNA cleavage into oligonucleosomal-sized fragments was also observed (Figure 4, lower panel).
Figure 3
Representative cytometric analyses of apoptotic melanocytes (upper panel), and percentage of apoptotic cells (lower panel). HEMn-LP and HEMn-DP were exposed to various concentrations of SPER/NO for 24 h. Then, the cells were double-stained with CF488A-Annexin V and propidium iodide, and analysed by flow cytometry. Images display the distribution of viable cells (lower left quadrant), early apoptotic cells (lower right quadrant), late apoptotic cells (upper right quadrant), and necrotic cells (upper left quadrant) expressed as a percentage
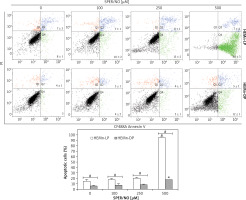
Figure 4
Cell apoptosis observed using Hoechst 33342 staining and DNA fragmentation. The morphology of HEMn-LP and HEMn-DP nuclei grown for 24 h without (control) and with 250 and 500 μM SPER/NO revealed by fluorescence microscopic detection (magnification: ×400). Chromatin condensation and nucleus fragmentation are indicated by arrows (upper panel). DNA fragmentation in HEMn-LP (lane 1) and HEMn-DP (lane 3) treated with 500 μM SPER/NO for 24 h. Lanes 2 and 4 – untreated HEMn-LP and HEMn-DP, respectively; lane 5 – positive control (apoptotic U937 cells); lane 6 – DNA ladder marker (lower panel)
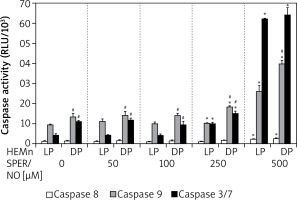
Culturing of HEMn in the presence of 250 and 500 μM SPER/NO led to the significant activation of caspases 9 and 3/7 (Figure 5). The highest concentration used caused a nearly 3-fold increase in caspase 9 activity in both HEMn types. Under the same conditions, caspase 3/7 activity increased 6-fold in HEMn-DP and as much as 14-fold in HEMn-LP, as compared to the untreated control cells. Relatively low activity of caspase 8 was slightly elevated only in the melanocytes growing with 500 μM SPER/NO.
Figure 5
Caspase activation by nitric oxide in HEMn-LP and HEMn-DP. Melanocytes were treated with indicated SPER/NO concentrations (50–500 μM) and cultured for 24 h. Activities of caspase 9, caspase 3/7, and caspase 8 were determined as described in Material and methods. Data are the mean ± SEM of at least 3 independent experiments; *p < 0.05 vs. untreated control, #p < 0.05 between HEMn-LP and HEMn-DP
Nitric oxide affects BCL-2 and BAX expression
As shown in Figure 6, SPER/NO treatment had no effect on BAX expression in HEMn-LP. In HEMn-DP, the exposure to NO donor increased the BAX mRNA level (3-fold compared to the control) only at 500 μM. Up to 250 μM, SPER/NO had no significant effect on BCL-2 expression in both types of HEMn, whereas exposure of cells to the highest concentration of the NO donor (500 μM) led to a 1.5-fold and 6.5-fold increase in BCL-2 expression in HEMn-LP and HEMn-DP, respectively.
Figure 6
Relative BAX and BCL-2 mRNA expression levels in HEMn-LP and HEMn-DP treated with various SPER/NO concentrations (50–500 μM) for 12 h. The mRNA transcript levels of BAX and BCL-2 gene were measured by real-time reverse transcription-polymerase chain reaction. Fold changes in BAX and BCL-2 mRNA expression levels were calculated relative to the untreated control cultures
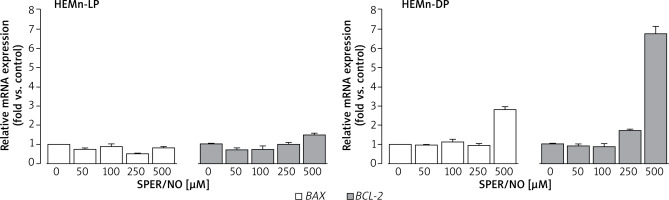
In general, DP melanocytes cultured with 0-250 μM SPER/NO showed 2-fold higher expression of BAX and BCL-2 than LP melanocytes grown under the same conditions (Table 1). The difference in the apoptotic gene expression between the cells was even more pronounced at the highest concentration of the NO donor. In this case, BAX and BCL-2 mRNA levels in HEMn-DP were 3-fold and 5-fold higher than in HEMn-LP, respectively.
Table 1
Fold changes in BAX and BCL-2 mRNA expression levels refer to the comparison of HEMn-DP with HEMn-LP. Data represent the mean ± SEM (n = 3)
| SPER/NO [μM] | Relative mRNA expression (HEMn-DP vs. HEMn-LP) | |
|---|---|---|
| BAX | BCL-2 | |
| 0 | 1.6 ±0.1 | 1.9 ±0.02 |
| 50 | 2.1 ±0.03 | 2.5 ±0.23 |
| 100 | 2.0 ±0.24 | 2.4 ±0.35 |
| 250 | 1.6 ±0.18 | 1.7 ±0.06 |
| 500 | 2.9 ±0.17 | 4.6 ±0.25 |
Discussion
Our results demonstrate that NO is capable of inducing apoptosis in normal human epidermal melanocytes. The cell response to NO depends on its concentration in the culture medium and seems to be modulated by the pigmentation phenotype of the cells. As a source of NO we used SPER/NO, which spontaneously decomposes when added to the culture medium, liberating 2 moles of NO per mole of the parent molecule [21, 22]. Such a method of delivering NO to the culture system mimics to some extent the pathophysiological processes in the skin, where melanocytes are stimulated in an autocrine or paracrine manner by extracellular NO.
Microscope assessment of HEMn growing in the SPER/NO-supplemented media suggested that NO at high concentrations was toxic to the cultured cells. When the cells were cultured in the presence of 250 and 500 μM SPER/NO, we observed changes in cell shape and loss of adherence. Our preliminary conclusion about NO toxicity towards HEMn was confirmed by the results of the cell proliferation and viability assays. After exposure to 250 μM SPER/NO, the number of actively proliferating HEMn-DP in relation to the matched control was significantly lower than that estimated for HEMn-LP. Doubling the concentration of the NO donor almost completely blocked HEMn proliferation and markedly reduced relative cell viability. Interestingly, the viability of HEMn-DP treated with 500 μM SPER/NO was significantly higher than HEMn-LP cultured under the same conditions. Although the cell viability assay used here, based on measuring of total cellular ATP levels, does not distinguish between cytotoxic and cytostatic effects, the cell detachment from the monolayer suggested cell death as a result of NO treatment. Indeed, we found that NO released from SPER/NO induced cell shrinkage, chromatin condensation, nuclear fragmentation, and degradation of genomic DNA in cultured HEMn-LP and HEMn-DP, which are the morphological and biochemical hallmarks of apoptosis. As shown by flow cytometric analysis, the presence of NO in the culture medium increased the percentage of apoptotic HEMn compared with the untreated controls, especially at the highest concentration of the NO donor, where the increase was found to be significant. HEMn from darkly pigmented skin were much more resistant to apoptotic death than those from lightly pigmented skin. At 500 μM SPER/NO, almost all of HEMn-LP in culture were at the stage of early or late apoptosis, whereas the percentage of apoptotic HEMn-DP was significantly lower.
Significantly elevated caspase 9 activities compared to the controls indicates that NO exposure primarily activates the intrinsic (mitochondrial) apoptosis pathway in the cultured HEMn. Again, the cell response was found to be associated with the pigmentation phenotype. Interestingly, although HEMn-DP responded more strongly than HEMn-LP in this regard, the activation of the effector caspase 3/7 under high concentrations of NO was proportionally weaker in HEMn-DP than in HEMn-LP. The activation of the intrinsic pathway of apoptosis was described earlier for human foreskin melanocytes exposed to UV radiation [23]. Our results show that, at high concentrations, NO can also activate the extrinsic (death receptor) apoptotic pathway in HEMn, as indicated by slightly increased caspase 8 activity in the cells cultured in the presence of 500 μM SPER/NO. It should be noted, however, that caspase 8 can also be activated by caspase 9 via caspases 7 and 6 [24]. A key role in the regulation of the intracellular apoptosis pathway is played by proteins of the Bcl-2 family, which includes proteins that both inhibit (Bcl-2, Bcl-xL) and/or initiate cell apoptosis (Bax, Bcl-xS, Bak, Bik, Bim, Bad, Bid) [25]. We found that NO exposure did not affect the mRNA expression of either BAX or BCL-2 in cultured HEMn-LP, even at the highest concentration of the NO donor that led to the significantly enhanced expression of both the genes in HEMn-DP. Importantly, the relative expression of antiapoptotic BCL-2 in HEMn-DP versus control increased almost 7 fold, whereas proapoptotic BAX only 3 fold. This could explain why HEMn-DP are more resistant than HEMn-LP to apoptosis induced by high concentrations of NO in the culture medium. Some studies on macrophages show that BCL-2 overexpression may be protective against NO-induced cytotoxicity. BCL-2 has been found to interfere upstream of caspases, i.e. caspase-3 activation, and therefore acts as an effective signal terminator during NO-induced apoptosis [26]. Dhakshinamoorthy et al. [27] indicated that BCL-2 successfully counteracts NO-induced death of neuroblastoma cells. Kim et al. [28] reported that a high constitutive level of BCL-2 protein can protect melanocytes from damage caused by UVB radiation. Bivik et al. [29] showed that after UVB exposure melanocytes up-regulated BCL-2 at the mRNA level without showing any change at the protein level. Our results are in line with these and other findings, which underscore an important role for the BCL-2 protooncogene and its protein product in the cell resistance to NO-induced apoptosis [30–32].
The results of the present study suggest that the pigmentation phenotype may be an important modulating factor responsible for the different response of human epidermal melanocytes to proapoptotic activity of extracellular NO. The commercially available cells we used here were derived from the donors of various degree of constitutive skin pigmentation. A previous study revealed that HEMn-DP contains almost twice as much melanin as HEMn-LP [33]. Stępień et al. [34] demonstrated in vitro that melanin can act as an effective scavenger of peroxynitrite, a highly reactive molecule that is readily formed from NO under oxidative stress conditions. It seems likely that intracellular melanin can also act as a natural scavenger of reactive nitrogen species. This could go some way to explaining why HEMn-DP are more resistant to the cytotoxic effects of NO released from SPER/NO and why apoptosis is less intense in these cells compared to HEMn-LP. Numerous studies have shown that there is a relationship between the effect of various cytotoxic agents and the degree of melanocyte pigmentation [35–40]. It was also shown that the cell pigmentation phenotype modulates the inflammation-induced expression of numerous genes encoding cytokines and chemokines in human epidermal melanocytes in culture. The proinflammatory response of HEMn-LP was generally more pronounced than that of HEMnDP [19]. On the other hand, the genetic background of a given melanocyte phenotype could also be involved in the cell response to various stimuli, including NO. Genome-wide transcriptome analysis revealed differences between melanocytes from dark and light skin in basal expression levels of some genes important for melanocyte development and functions [19, 41].
We believe that the results of our study may contribute to a better understanding of skin pathology, including vitiligo. Although the pathogenesis of melanocyte destruction characteristic of this depigmentation disorder is still under investigation, apoptosis is listed as one of the cell death modalities [42]. Our study suggests that NO, the level of which is known to be elevated in vitiligo lesions as a result of overexpression of NOS [43], could trigger epidermal melanocytes to undergo apoptosis, and that the cell response could be modulated to a great extent by the pigmentation phenotype.








