Introduction
Introduction of modern, highly effective immunosuppressive agents in treatment of organ transplant recipients (OTRs) has led to a significant reduction in post-transplant rejection rates and improvement of graft survival. Current potent immunosuppressive drugs are relatively safe however not devoid of side effects. Malignancy represents a major burden in transplantation medicine. Due to impaired immunity, cancers are more aggressive and may result in serious morbidity or mortality.
Non-melanoma skin cancers (NMSC) are the most common malignancies in solid organ transplant recipients [1–6]. Incidence rates of post-transplantation NMSC are approximately 7% after 1 year and 10–45% after 10 years since organ transplantation [3]. Chronic immunosuppression is the main cause of malignancies, however the type and duration of immunosuppression is also of great importance. Other risk factors associated with a higher incidence of NMSC are advanced age, male sex, fair skin phototype, skin cancers prior to transplantation, premalignant lesions, UV exposure, infections (HPV, EBV etc.) and also genetic disorders such as autosomal dominant polycystic kidney disease [4, 5]. Different treatment modalities can result in not only increased incidence of cancers but also impaired organ function, mainly kidney [7]. Unlike in the general population, the most common skin cancer in OTRs is cutaneous squamous cell carcinoma (cSCC) [4, 6]. Other malignancies also reported are: basal cell carcinoma (BCC), Kaposi sarcoma (KS), Merkel cell carcinoma (MCC) and malignant melanoma (MM). The risk of developing SCC in OTRs is increased by 65 to 250-fold compared to the general population [6].
Calcineurin inhibitors (CNIs), cyclosporine A (CsA) and tacrolimus (TAC), are effective immunosuppressants used in treatment and prophylaxis of graft rejection following organ transplantation which are well documented to contribute to an increased incidence of secondary skin cancers [8–10]. Changes of treatment regimens including dose minimization and drug alterations seem to reduce development of skin cancers in OTRs. SRL and EVR are inhibitors of the mammalian target of rapamycin (mTORi) that were reported to have skin carcinoma preventive properties [11–17].
Aim
The aim of this review was to analyse the available medical literature on the incidence of skin cancers after conversion from CNIs to therapy with mTORi in kidney transplant recipients.
Material and methods
An extensive search in the literature was performed in line with the criteria published in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [18] guidelines by two investigators using Medline and Web of Science electronic databases. We used the following combination of searched key words: “sirolimus”, “everolimus”, “rapamycin”, “conversion”, “randomized controlled trials”, “renal transplant” and “kidney transplant”. Inclusion criteria: articles concerning incidence of NMSC among transplant recipients converted from CNIs to mTORi, adult patients, and no duplicated data in the study. Articles not including non-melanoma skin cancers, treatment regimen without conversion from CNIs to mTORi, children, reviews, case reports, clinical trials not being RCTs and personal experience summaries, studies not meeting the inclusion criteria of this study or in a language other than English were excluded. Relevant articles were screened by title and abstract, then the full text was assessed for eligibility. The search was concluded in November 2021.
Results
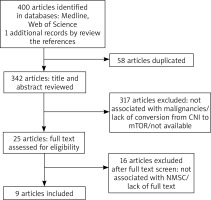
The flow diagram of this study is presented in Figure 1 [18]. We identified 400 potential studies and publications, and then 58 were excluded for duplication, 317 were excluded after title and abstract screening, while 16 were excluded after full text screening. Furthermore, articles were excluded for not being randomized controlled trials, not providing information on incidence of NMSC, not including NMSC, review articles, case reports or animal studies. The final set included 9 articles. Key studies and findings are highlighted in Table 1.
Table 1
Summary of published RCTs reporting the impact of conversion from calcineurin inhibitors to sirolimus/everolimus in renal transplant recipients on skin cancer incidence
| Study | Year | Sample size | Main conclusions |
|---|---|---|---|
| Campistol [31] | 2006 | Renal transplant recipients (n = 430) SRL-CsA-ST (n = 215) SRL-ST (n = 215) | |
| Salgo [17] | 2010 | Renal transplant recipients with premalignant skin lesions (n = 44) SRL (n = 25) Common therapy (CsA, TAC, azathioprine) (n = 19) | |
| Euvrard [15] | 2012 | Renal transplant recipients with at least one invasive SCC (n = 109) CNI (n = 56) SRL (n = 64) |
|
| Campbell [16] | 2012 | Renal transplant recipients (n = 86) CNI (n = 47) SRL (n = 39) | |
| Hoogendijk-van den Akker [11] | 2013 | Renal transplant recipients with at least one confirmed SCC (n = 155) SRL (n = 74) Other (n = 81) | |
| Alberu [12] | 2011 | Renal transplant recipients (n = 830) SRL (n = 555) CNIs (n = 275) |
|
| Tedesco-Silva [30] | 2016 | Renal transplant recipients: (n = 254) evaluable for safety (n = 195) evaluable for efficacy analysis: SRL (n = 86) CMI (TAC) (n = 109) | |
| Dantal [32] | 2018 | Renal transplant recipients with one SCC (n = 120) SRL (n = 64) CNI (n = 56) |
|
| Ying [33] | 2018 | Renal transplant recipients (n = 192) EVR + RD-CNI (n = 131) EVR + CNI-WD (n = 68) Controls: TAC/CsA + MPA + CS (n = 87) |
|
Impact of immunosuppressive drugs on the molecular mechanism of skin cancer
Lifelong immunosuppression of patients who underwent organ transplant is the primary but not solitary reason for increased frequency of malignancies. It seems that pathogenesis of skin malignancies involves complex interactions between OTRs’ genetic background, demographics, oncogenic viral co-infections, UV-induced effects and immunosuppressive agents [4, 8].
CNIs have been proven to induce initiation and promotion of skin cancers by their mechanism of action [19]. CsA seems to contribute to carcinogenesis and tumour progression by inhibition of calcineurin, resulting in activation of transcription factor 3 (ATF3). ATF3 belongs to the AP-1 family, which downregulates p53 expression by negative regulation of p53 messenger RNA expression [20, 21]. ATF3 was observed to increase formation of SCC in mice and in humans [20]. CsA causes also resistance to UV‐induced apoptosis and promotes growth factors [19, 22]. Moreover, UV light exposure is well known to be a major factor for SCC formation. Dziunycz et al. reported a synergistic effect of combined UV light and CsA treatment causing potentiated expression of ATF3 resulting in development of SCC in the sun-exposed areas of the OTRs’ skin [22].
However, CNIs constitute a crucial treatment option in OTRs which prevents post-transplant rejection, various therapeutic options have been extensively studied to find treatment regimens decreasing the rate of skin cancer in this population. Among the most often studied are mTORi which possess both immunosuppressive and antitumor activity [23, 24].
SRL and EVR are macrolide antibiotics the pharmacokinetic and drug interaction profile of which is similar to CNIs (CsA, TAC), however they have immunosuppressive, antineoplastic and antifungal properties. mTORi act through the mechanism of inhibition of a serine-threonine kinase, mammalian target of rapamycin. Moreover, they block the response of T- and B-cell activation by cytokines [24]. A few studies have reported a lower incidence of skin malignancies in transplant recipients treated with mTORi (Table 1). mTORi compared to CNI have a renoprotective effect, they cause less nephrotoxicity and prevent chronic allograft nephropathy [25, 26].
The differences between CNI and mTORi treatment results might be a matter of distinct impact on the function of regulatory T cells (Tregs) which are crucial for the induction and maintenance of peripheral tolerance. CNIs suppress both cytotoxic cells and Tregs functions, while mTORi seem to preserve the survival of Tregs resulting in tolerance towards alloantigens in organ transplant recipients [27]. Cegielska et al. observed that mTORi increase the level of Tregs in peripheral blood in kidney transplant recipients suggesting that mTORi allow to reduce the immunosuppression needed after organ transplantation [27].
On the other hand, mTORi are not deprived of adverse effects. Both adverse events (AEs) and serious adverse events (SAEs) have been observed after conversion to SRL/EVR. The leading AE include oral mucositis (aphthous ulcers), acneiform skin lesions, elevated transaminases, increase in triglycerides and cholesterol levels [28, 29]. Other frequent side effects are pneumonitis, proteinuria, diarrhoea and oedema [16, 17]. Moreover, patients converted to mTORi have relatively high discontinuation rates related to AEs but also higher rates of acute rejection compared to CNI.
Review of clinical trials
A summary of published studies reporting conversion from CNIs to SRL/EVR in kidney transplant recipients and its influence on skin cancers is presented briefly in Table 1. Multiple clinical trials presented a reduced risk of different types of NMSCs after CNI withdrawal and conversion to SRL/EVR after organ transplantation.
Campbell et al. performed a study that examined 86 transplant recipients with a history of NMSC that continued CNI (n = 47) or were converted to SRL (n = 39) [16]. The rate of new biopsy confirmed NMSC lesions per patient year was significantly lower in the SRL group than the CNI group (1.31 vs. 2.48, p = 0.022). The rates of new SCCs (0.88 vs. 1.77, p = 0.038) were also significantly lower in the group. However, in the BCC groups, no significant difference was found (0.43 vs. 0.77, p = 0.104) [16]. Discontinuation rates related to AEs were significantly higher for SRL than CNI (46.2% vs. 0%; p < 0.001) [16]. Of note, there was a high rate of study discontinuation in the SRL group (79%) while it was 49% in the control group.
Regarding efficacy of mTORi in skin cancer prevention, TUMORAPA trial by Euvrard et al. compared kidney transplant recipients on CNI therapy with patients who switched to SRL in terms of the anti-tumoral effect and history of previous invasive SCC [15]. The SRL group had 64 patients and 56 patients continued CNI therapy. Both groups had a history of a single cutaneous SCC (55% of patients of each group) and multiple lesions (45% of each group). The SRL group had a significantly longer survival free of cutaneous SCC than patients in the CNI group, with the hazard ratio for new SCC of 0.37 (95% confidence interval (CI): 0.16–0.85), and a study-adjusted hazard ratio of 0.38 (95% CI: 0.17–0.84) [15]. However, this difference remained significant only for the patients with a single SCC. Skin cancers developed in 20 patients in the SRL group and in 31 in the CNI group (47.6% vs. 70.5%, p = 0.048) [15]. AEs were more frequent in the SRL group than the CNI group and led to discontinuations in 15 (23%) patients after a median of 2.5 months. Interestingly, patients converted to SRL with rapid protocols had a higher rate of discontinuation than those who were converted with progressive protocols. Moreover, it seems that SRL antitumor properties are efficient when it is introduced after the occurrence of single, rather than multiple cutaneous SCCs [15].
Hoogendijk-van den Akker et al. also examined impact of conversion to SRL in renal transplant recipients on the incidence of SCCs [11]. The study group included 155 patients, 74 in the SRL group and 81 in the control group. Patients were converted from azathioprine, mycophenolate mofetil, CsA and/or TAC to SRL. In contrast to previous studies, Hoogendijk-van den Akker et al. demonstrated a statistically significantly lower rate of invasive SCC after 1 year of treatment (significant 50% risk reduction of a new SCC) (hazard ratio = 0.50 (95% CI: 0.28–0.90; p = 0.007)), while it failed to show benefits in terms of SCC-free survival at 2 years (non-significant 24% reduced risk), (hazard ratio = 0.76 (95% CI: 0.48–1.2; p = 0.255)) [11]. Moreover, SRL was especially effective during the first year after conversion in patients with only one previous SCC. Only one of 30 patients developed a new SCC 9 months after conversion in this group compared with 6 of 23 patients in the control group (p = 0.015) [11]. Twenty-nine (29) converted patients (39.1%) discontinued treatment because of AEs after a median time of 5.6 months. Interestingly, again SRL was proved to be much more effective during the first year with only one previous SCC compared with multiple SCCs before inclusion [11]. Those results are in line with Euvrard et al. study indicating that mTORi conversion is beneficial in patients with only one previous invasive SCC [15]. Moreover, increased incidence (39%) of AEs in patients converted to SRL was observed, compared to patients (2.5%) without conversion therapy. A high discontinuation rate was observed (42%) and it was similar to previous studies (24–35%) [15, 16].
A randomized controlled trial performed by Salgo et al. examined the impact of conversion to sirolimus on the rate of premalignant skin lesions and NMSC in renal transplant recipients [17]. The study examined 44 renal transplant patients who either continued previous immunosuppressive medication (n = 19) (azathioprine/mycophenolate, CsA, TAC) or switched to SRL (n = 25) [17]. It showed that conversion to SRL in renal transplant patients inhibited the progression of premalignancies and significantly decreased the number of patients who developed histologically confirmed NMSC (6.3% vs. 47.1%; p = 0.017) during 1-year follow-up compared to the control group [17]. Interestingly, the SRL group showed a high discontinuation rate of 36% compared to 11% in the control group [17].
Since most of conducted trials had short follow-up, usually lasting 1 year, efficacy of antineoplastic properties of SRL were questionable in terms of lifetime therapy. Nevertheless, results of recent studies have resolved doubts. Some clinical trials examined malignancy rates in post-renal-transplantation maintenance therapy with a longer follow-up.
Tedesco-Silva et al. analysed patients after kidney transplantation with early conversion to SRL during 24 months [30]. As seen in other studies, conversion to SRL was associated with fewer cases of NMSC compared with CNI (TAC) continuation (0 vs. 6; p = 0.012) [30]. However, withdrawal from CNI and switch to SRL was associated with higher rates of biopsy-confirmed acute rejection (8% vs. 2%; p = 0.02) and discontinuation due to adverse events (21% vs 3%; p < 0.001) [30].
Hoogendijk-van den Akker et al. found that conversion to sirolimus resulted in a lower rate of SCC after 1 year of treatment but it failed to show beneficial outcomes after 2 years [11]. On the other hand, Alberu et al. study showed that patients undergoing SRL therapy had significantly lower rates of NMSC through 2 years post-conversion than the CNI group (1.2 vs. 4.3, p < 0.001). However, the rate of other cancers (excluding skin cancers) was not significantly different between treatment groups (p = 0.058) [12]. It seems that reduced rates of cancer after conversion from CNIs to SRL are driven by skin cancers rather than other cancers.
Another interesting trial by Campistol et al. compared two treatment groups that both contained SRL (SRL-CsA-steroid (ST) vs. SRL-ST after CsA withdrawal at month 3) over a longer period of time [31]. The median time to first skin carcinoma was delayed, moreover the risk of skin carcinoma was significantly lower with SRL-ST therapy (SRL-ST to SRL-CsA-ST 0.346; 95% confidence interval 0.227 to 0.526; p < 0.001, intention-to-treat analysis). The results showed also reduced incidence of non-skin malignancies (9.6% vs. 4.0%; p = 0.032) and a significantly lower risk for an event at 5 years after renal transplantation compared with those who received SRL therapy combined with CsA (relative risk SRL-ST to SRL-CsA-ST 0.346; 95% confidence interval 0.227 to 0.526; p < 0.001) [31].
The research results of Dantal et al. who extended at 5 years the TUMORAPA randomized trial are very promising in terms of long-time therapy [15, 32]. Dantal et al. study proved that conversion to SRL significantly decreases the risk of cutaneous carcinomas (not only SCC) and the beneficial effect is maintained at 5 years [32]. The number of patients with new skin cancers was significantly lower in the SRL group compared with the CNI group: 22% vs. 59% for SCC (p < 0.001), 34% vs. 66% for other skin cancers (p < 0.001), and 20% vs. 37.5% for basal cell carcinomas (p < 0.05) [32]. Moreover, survival free of cutaneous SCC was significantly longer in the SRL group than in the CNI group (p = 0.007) [32]. However, this result remained statistically significant only among patients with a single SCC before randomization, which is in line with other randomized controlled trials [11, 15, 16]. AEs were frequent, similarly to previous studies. Almost all patients had drug-related AEs that led to discontinuation in 23% of patients within 2 years and additionally 11% of patients between 2 and 5 year of trial [32]. The rate of discontinuation was similar to other studies [11, 16]. No episode of acute rejection occurred in the SRL group and the renal function was stable during the trial [32]. Both Campistol and Dantal et al. studies showed long-term anti-tumoral effect of conversion from CNIs to SRL that was maintained at 5 years [31, 32].
A recent study by Ying et al. for the first time compared such a long-term risk (9-years) of cancer, NMSC incident and death attributed to cancer among kidney transplant recipients [33]. Patients were randomized to de novo, early conversion EVR regimen or CNI-based triple therapy. EVR use was not associated with a reduction in the 9-year risk of incident cancer or cancer-related death compared with standard CNI-based therapy. Incidents of cancer were similar in both groups, 51 (26.6%) in the everolimus group and 21 (24.1%) in the control group (p = 0.6) [33]. Moreover, the proportion of cancer deaths between EVR and control patients was similar (everolimus = 4.7%, control = 3.4%; p =1.0) [33]. Interestingly, compared to controls only patients randomized to everolimus + reduced-dose calcineurin-inhibitor showed a 56% reduction for NMSC (unadjusted HR = 0.44 (0.21–0.92)), which remained significant after adjusting for age, gender and smoking (adjusted HR 0.45 (0.21–0.96)) [33]. The reduction in the risk of NMSC was not seen in the CNI-withdrawal group. Unlike previous studies, Ying et al. showed that a combination of mTOR inhibitor and CNI in a reduced dose is beneficial in terms of the long-term risk of NMSC compared to mTOR and CNI withdrawal.
Disadvantages of conversion from CNIs to SRL/EVR in OTRs
A regimen of SRL alone may be advantageous for decreased incidence of skin malignancies however multiple studies reported the low allograft survival, more common SAEs and higher mortality rate related to mTORi conversion [34–38].
Knoll et al. meta-analysis of 5876 kidney transplant recipients from 21 randomized trials confirmed that SRL was associated with reduction in the risk of NMSC (56%) and all types of malignancy (40%) [34]. The large study group enabled to detect that SRL was associated with an increased risk of death, both in the conversion (p < 0.001) and de novo trials (p = 0.003) [34].
Another large longitudinal cohort study analysing data from the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry, included 9353 adult patients who underwent kidney transplants, and confirmed a higher mortality risk with the use of mTORi while the treatment was not significantly associated with the risk of graft loss [35]. Meta-analysis of 29 randomized trials, likewise, demonstrated that discontinuation secondary to AEs was more common in patients on mTORi than on CNI (21.6% vs. 9.6%, p = 0.020) [38]. Moreover, patients converted to mTORi had a higher risk of acute rejection within the first year following the conversion (p = 0.330) [38].
The increased mortality observed in multiple studies [34–37] seems to diminish the decreased risk of cancer incidence during mTORi therapy. The results point out a need for a review of indications for CNI elimination and conversion to mTORi in transplant recipients. When it comes to a high rate of graft rejection and discontinuation of treatment due to AEs related to mTORi conversion, Tedesco Silva et al. study showed that EVR with reduced CsA might be a remedy [39]. Such treatment combination enables reduction of the CNI dose resulting in lower nephrotoxicity, NMSC incident and graft dysfunction but also minimizes the risk of graft rejection observed in conversion to mTORi. Tedesco Silva et al. proved that EVR plus reduced-exposure CsA is as effective as mycophenolic acid (MPA) plus standard-exposure CsA in terms of safety and efficacy but also the incidence of AEs is comparable [39].
Summary
Multiple studies have shown that conversion to mTORi from CNI in post-renal transplantation patients reduces the risk and delays the occurrence of NMSC. However, mTORi seems not to be a remedy in every case. Unless, the conversion of mTORi occurs after an initial diagnosis of a single cutaneous SCC it fails to show benefits in terms of multiple SCCs. Moreover, reduced rates of cancer were observed in skin malignancies rather than other cancers. Short follow-up of initial trials with SRL raised the subject of its efficacy in long-time therapy, however recent studies have confirmed that SRL beneficial effects were maintained even at 5 years. In spite of mTORi anti-tumoral effects, conversion to SRL seems to be associated with many disadvantages. In comparison to CNI, mTORi present increased incidence of AEs which usually lead to treatment drop out. Therefore, discontinuation rates are much higher in mTORi compared with CNIs. Despite a reduced risk of cancers, mTORi fail to show improved patients’ survival. A few recent studies highlighted the increased risk of death associated with SRL use which seems to diminish its beneficial effects as an immunosuppressive agent in transplant recipients. Nevertheless, majority of clinical trial results indicate a clinical benefit from the conversion to a mTORi-regimen in patients with low tumour burden in the early stage of disease. High AE rates and discontinuation of mTORi therapy results in search for alternative treatment strategies. Recent trials showed that efficacy and safety of EVR with a reduced dose CNI is comparable to standard treatment with CNI-MPA. Moreover, such treatment regimen resulted in reduction of the risk of developing NMSC, without the risk of graft rejection, therefore being a promising alternative to conversion from CNI to mTORi. It seems that further studies are needed to determine who would benefit from mTORi conversion or mTORi combined with reduced-dose CNI.