Introduction
Congenital melanocytic nevi (CMN) are hamartomas, benign proliferative lesions composed of clonal proliferations of melanocytes, which occur during embryogenesis due to mutations. By definition, CMN are present at birth or begin to appear usually over the course of a few months of life [1, 2]. The names applied to congenital nevi reflect the largest estimated diameter of the nevus in adulthood: small (< 1.5 cm), medium (1.5–20 cm), large (> 20–40 cm), and giant (> 40 cm) [3]. The estimated incidence of CMN is as follows: small in 1 in 100 births, medium in 1 in 1000, large in 1 in 20,000, and giant in 1 in 500,000 [4]. Small and medium congenital melanocytic nevi are common lesions and generally remain benign throughout the lifetime of a person, and the risk of developing a melanoma is considered low [5]. Notwithstanding, the vast majority of studies focus on complications in large and giant CMN. There are a limited number of studies focused on the risk of melanoma in medium-sized congenital melanocytic nevi.
Aim
The aim of this article is to contribute to the expansion of knowledge in this area by publishing our analysis of surgically removed medium-sized CMN in the Unit of Dermatosurgery between 2016 and 2020. We conducted this study to optimize clinical care for patients with medium-sized CMN, by gathering more information due to the limited available data.
Material and methods
This retrospective study included 10 patients, who were operated on in the Unit of Dermatosurgery between 2016 and 2020. All participants presented with non-multiple medium-sized congenital melanocytic nevus. After dermatological examination, dermoscopy, and clinical evaluation, the decision about surgical excision was made. According to the location and size of the nevus, the technique of simple or serial excision was performed. The procedures were carried out after obtaining informed consent from the patients.
Results
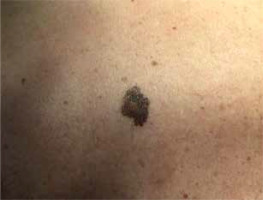
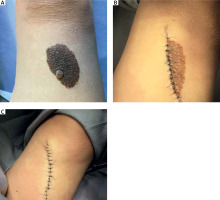
In the study group there were 3 men and 7 women. The average age of the patients was 33 ±16.1 years. The diameter of the presented medium-sized congenital melanocytic nevi ranged 20–70 mm (mean: 36.4 ±18.1 mm). The area of the lesion ranged 11.31–100.53 cm2 with a mean size of 36.45 ±31.73 cm2. In most of the cases the reason for excision of the medium-sized CMN was evolution of the lesion or aesthetic considerations reported by the patient. However, bleeding, itch, or nodule development within the congenital melanocytic nevus were also observed. In 2 cases, due to the large size of the lesions, serial excisions were performed, while other CMN were removed surgically using a simple excision technique. Figure 1 shows a 2-step excision of a medium-sized CMN on the right thigh of a 68-year-old woman. The other case presents serial excision of a medium-sized CMN on the left forearm of a 26-year-old man (Figure 2). Possible malignant transformation to melanoma prompted the decision to perform excision along lymphatic drainage. Eight of ten medium-sized CMN were histologically described as benign, and 2 cases of malignant transformations were reported. The patient presenting with a 25-mm CMN located on the back, accompanied by bleeding and itching, was diagnosed with melanoma (Clark III, Breslow 0.8 mm) on the basis of histological examination (Figure 3). The second diagnosed melanoma (Clark I, Breslow 0.2 mm) arose on a 25-mm CMN on the trunk without any obvious clinical signs of malignancy other than evolution of the lesion reported by the patient. Table 1 summarizes the baseline characteristics of the patients.
Table 1
Cases of surgically removed medium-sized CMN in the Unit of Dermatosurgery in the years 2016–2020
| No. | Age | Sex | CMN location | Maximum clinical diameter [mm] | Size [cm2] | Reason for excision | Histological diagnosis | Type of operation |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | M | Trunk (back) | 25 | 18.06 | Bleeding from the lesion, itching | Melanoma, Clark III, Breslow 0,8 mm | Simple excision with 3 mm margin |
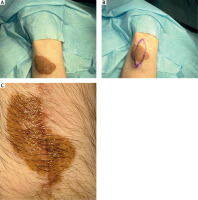
| 2 | 68 | F | Right thigh (Figure 1) | 70 | 76.97 | Small tumour growing on the basis of the lesion | CMN (dermal nevus) | Two-step excision |
| 3 | 19 | F | Shank | 20 | 11.31 | Itching, aesthetic reason | CMN (dermal nevus) | Simple excision |
| 4 | 22 | F | Left buttock | 30 | 20.73 | Evolution of the lesion according to the patient | CMN (dermal nevus) | Simple excision |
| 5 | 44 | M | Trunk (back) (Figure 3) | 25 | 15.71 | Evolution of the lesion according to the patient | Melanoma, Clark I, Breslow 0.2 mm | Simple excision |
| 6 | 26 | M | Left forearm (Figure 2) | 64 | 100.53 | Evolution of the lesion according to the patient, aesthetic reason | CMN (compound nevus) | Two-step excision |
| 7 | 18 | F | Right arm | 50 | 62.83 | Evolution of the lesion according to the patient, aesthetic reason | CMN (compound nevus) | Simple excision |
| 8 | 17 | F | Right leg | 25 | 14.14 | Evolution of the lesion according to the patient, aesthetic reason | CMN | Simple excision |
| 9 | 34 | F | Lower back | 30 | 20.73 | Evolution of the lesion according to the patient, aesthetic reason | CMN (compound nevus) | Simple excision |
| 10 | 42 | F | Abdomen | 25 | 16.49 | Evolution of the lesion according to the patient | CMN (compound nevus) | Simple excision |
Figure 1
Serial excision of medium-sized congenital melanocytic nevi (CMN) on the right thigh. A – Preoperative view, B – after the first excision, C – after the second excision
Discussion
The risk of development of cutaneous melanomas within CMN is strongly associated with the size of the lesions. Cutaneous melanoma most likely arise on large and giant CMN [6]. For years malignant transformations of medium-sized CMN have been controversial [7].
It is worth noting that there is limited research evaluating such outcomes in the literature, and there is a need for increased knowledge on this topic. The most common location of medium-sized CMN is a lower limb [7]. However, the back is the most common site for CMN-associated melanomas [8]. There is no sex, ethnic group, or race predilection [9]. More than two-thirds of medium-sized CMN and a small subset of melanomas harbour somatic gain-of-function mutations in NRAS, which result in cellular proliferations and explain some pathways of cancerogenesis [10].
In 2010 Price et al. estimated the risk of small- and medium-sized CMN-associated melanomas to be less than 1% [10]. To emphasize the rarity of melanoma arising on small- and medium-sized CMN, scientists quote 3 substantial cohort studies of 680 people highlighting that no one was diagnosed with melanoma during a mean of 13.5 years of monitoring. One retrospective cohort study was based only on medium-sized CMN [10]. A total of 227 patients were monitored for a mean of 6.7 years, and no melanoma was reported. Two other cohort studies were retrospective and included all sizes of CMN. However, the authors divided CMN into groups on the basis of size. In the first study of 214 patients, no melanoma arose on 1–19 cm CMN during a mean 9.2 years of monitoring. The second research was based on 239 cases, and after a median follow-up time of 24 years no melanoma developed on 1–19 cm CMN [10]. In 2005 Zaal et al. [11] made a retrospective population-based study including 3929 patients with CMN, who were monitored for a mean of 4.7 years [11]. Eleven cases of malignant transformations were reported within ≤ 20 cm CMN, and no further detailed size-division was made [11]. In 2020 Caccavale et al. [8] analysed their collected data and performed retrospective research on all histologically confirmed melanomas over a 14-year period with the aim to evaluate the commonness of CMN-associated melanomas. In their database of 2159 melanomas 27 of them were associated with CMN (1.25%), and 5 of them with medium-sized CMN (0.23%). It is important to highlight that the most common CMN associated with melanoma turned out to be small (21; 0.97%) followed by medium-sized CMN [8]. Although malignant transformations occur more frequently within large and giant CMN, the incidence of small- and medium-sized CMN is much higher, which explains the foregoing results. In 2020 Cuevas et al. performed retrospective research to investigate melanoma predictors in dermoscopy. Their database consists of 119 skin tumours, 87 (73.1%) of which were histologically diagnosed as small- and medium-sized CMN and 32 (26.9%) as small- and medium-sized CMN-associated melanomas [12].
The true risk of malignant transformation of medium-sized CMN is nearly impossible to calculate with any degree of confidence. It could only be provided by long-lasting and large prospective research of untreated CMN. Furthermore, there are insufficient available data to assess an approximate estimate of the risk due to small study sizes, often a retrospective nature of the research, not considering medium-sized CMN, or combining small- and medium-sized CMN in one study group. However, according to available databases, the possibility of development of melanoma within medium-sized CMN is a fact, and considering the higher incidence of medium-sized CMN compared to large and giant CMN, we draw the conclusion that it can occur in a clinical setting.
Although malignant transformation of medium-sized CMN can occur, the magnitude of the risk remains unclear, and consistent recommendations for medical management do not exist. We do not routinely perform complete surgical removal as a prophylaxis against the development of melanoma. According to our clinical experience and knowledge, we recommend managing patients on an individual basis, taking into consideration multiple clinical attributes such as ease of monitoring, psychosocial complications, and aesthetic and functional concerns [3].
The presence of CMN may have an adverse impact on psychosocial well-being and can decrease quality of life [13, 14]. Therefore, the psychological impact of CMN should not be underestimated, and it can be a sufficient indication to perform surgery. In other cases, we recommend observation: regular visual inspection, baseline photographs, and dermoscopic follow-up.
Melanoma in solitary medium-sized CMN usually develops during or after puberty at the dermo-epidermal junction. On this account, dermoscopy is a crucial tool for follow-up and early melanoma detection [11, 15, 16]. Dermoscopy of congenital MN and acquired MN reveals similar features [17]. The dermoscopic pattern most likely to be visualized in medium-sized CMN is globular-reticular [18]. Macroscopically eccentric location of suspicious areas within CMN is an indicator of melanoma in small- and medium-sized CMN (333.3-fold increased risk) [12, 19]. In 2020 Cuevas et al. proposed dermoscopic predictors of melanoma arising in small- and medium-sized congenital nevi. Negative network (106.5-fold), grey angulated lines (12.5-fold), and atypical network (6.6-fold) were the dermoscopic features most associated with the development of melanoma [12].
However, well known factors that influence ease of monitoring with the naked eye and dermoscopy may influence the clinical approach: site of lesions, pigmentation, topography, thickness, hair presence, and non-cooperative patients [10].
Other clinical attributes such as CMN syndrome risk and extracutaneous melanoma risk are important in terms of management of CMN. Congenital melanocytic nevi syndrome is referred to as CMN associated with extracutaneous features, most commonly abnormalities in the central nervous system, i.e. neurocutaneous melanosis [20]. Risk factors for CMN syndrome include the presence of more than 2 medium-sized CMN. Children with CMN syndrome have significantly increased risk of extracutaneous and cutaneous melanoma associated with CMN size and abnormalities noted on screening MRI performed during the first years of life. Melanoma in the above-described subpopulation of patients is extremely aggressive with a high fatality rate [16]. Our research is based on solitary medium-sized CMN; therefore, the risk of malignant transformation within multiple medium-sized congenital melanocytic nevus will not be further discussed.
Tromberg et al. rightly pointed out that the main goal of CMN treatment is to avoid the development of melanoma while optimizing the aesthetic and functional outcomes [21].
Besides surgical excision, a wide range of treatment modalities such as dermabrasion, curettage, chemical peel, and laser treatments are available. However, all non-excisional techniques may be indisposed to early clinical diagnosis of melanoma. The surgical treatment methods included reconstructive ladder from direct excision with primary closure (single procedure or serial excision), skin grafting, expanded and unexpanded flap reconstruction, and free tissue transfer (in selective complex cases) [21].
The choice of procedure depends on the CMN size and the planned cosmetic effect. Moreover, some treatment modalities are more effective in different body regions. In a case of medium-sized congenital melanocytic nevus Yordanov et al. indicated local Dufourmentel skin flap as a reliable solution for surgical treatment of nevi located on the limbs [22]. Furthermore, staged excision has been favoured over single procedures for larger CMN. It is believed that multiple procedures give the skin a chance to stretch gradually, which possibly guarantees better outcomes [23]. Moreover, a retrospective review performed on 119 cases of small to medium congenital melanocytic nevi showed that treatment of small to medium CMN with excision followed by scar laser showed the highest IGA (Investigator’s Global Assessment) score, comparable to other treatment options. On the other hand, multiple surgical procedures may lead to the following complications: erythema, hypertrophic scarring, or widening of operation sites. Therefore, additional sessions with usage of a laser may provide more satisfaction for the patients [24]. It is also worth underlining that in the majority of cases a scar is socially more acceptable than a nevus [24, 25]. Summing up, for surgery of solitary, medium-sized CMN we utilize simple or staged total excision depending on the nevus size, to achieve the best cosmetic results and minimize scarring.
Conclusions
Malignant transformation of medium-sized CMN is a rare event, but in conjunction with an estimated incidence of medium-sized CMN of 1 in 1000 births, it can appear in clinical settings. Further studies in numerous samples are necessary to support any particular course of action for medium-sized CMN. In our opinion, long-lasting observation is the management of choice, and if there is need for surgery, we recommend total simple or staged excision depending on the nevus size.