Introduction
Alagille syndrome, also termed as syndromic bile duct paucity or arteriohepatic dysplasia involving the liver, heart, eyes, face and skeleton, is an autosomal dominant disorder due to mutations of the Jagged1 (JAG1) gene of the Notch signaling pathway [1]. The estimated prevalence of Alagille syndrome is 1 in every 100,000 live births [2]. The diagnostic criteria of Alagille syndrome are cholestatic jaundice, peripheral pulmonary artery stenosis, skeletal or ocular abnormalities, typical facial features and paucity of the bile duct on liver biopsy [3]. Cholestasis develops within the first 3 years of age with 45% of cases appearing in the first 3 months of life. The cholestasis may be associated with pruritus, episodic jaundice, hepatomegaly, xanthomas and high cholesterol, phospholipid and triglyceride levels [4]. Heart defects are found in 87.8% of cases [5]. The most common vascular manifestation of Alagille syndrome is pulmonary artery stenosis [6]. The pulmonary artery stenosis is usually peripheral, but sometimes central, valvular, supravalvular, or even hypoplastic or atresic [4]. Local peripheral pulmonary artery stenosis accounts for 75–90%, while diffuse pulmonary artery stenosis and pulmonary artery hypoplasia are uncommon, but capable of causing pulmonary artery hypertension [7]. Complex congenital heart defects, such as tetralogy of Fallot and pulmonary atresia, have been described in Alagille syndrome, which are proven to be associated with increased morbidity and mortality [7].
Aim
The aim of the present study is try to disclose the clinical features, management and outcomes of pulmonary artery stenosis associated with Alagille syndrome.
Material and methods
Literature retrieval from the PubMed, Google Scholar and “Baidu” Scholar was conducted for articles published from 1990 to 2021. The retrieval terms included “Alagille syndrome”, “syndromic bile duct paucity”, “arteriohepatic dysplasia”, “pulmonary artery”, “stenosis”, “atresia” and “hypoplasia”. The inclusion criteria were prospective or retrospective studies, case series and case reports of Alagille syndrome with the above-mentioned pulmonary artery pathologies. The primary exclusion criteria were publications concerning: patients with no substantial information (n = 7), Alagille syndrome with no involvement of the pulmonary artery (n = 3), Alagille syndrome with the involvement of the pulmonary artery but no information of pulmonary artery was available (n = 2), patients with pulmonary artery stenosis without Alagille syndrome (n = 1), computed tomographic imaging of the pulmonary artery of Alagille syndrome (n = 1), visceral artery anomalies in patients with Alagille syndrome (n = 1), pulmonary artery diverticulum (n = 1), pulmonary artery stenosis in Moyamoya disease (n = 1), Williams syndrome (n = 1) and duplicate publication (n = 1). The retrieval strategy is shown in Figure 1. As a result, a total of 38 articles were included and 19 articles were excluded [3–5, 8–42].
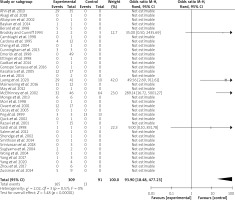
IBM SPSS statistics version 22 software was applied for statistical analysis. The measurement data were expressed as mean ± standard deviation and were compared by independent t test, while the categorical data were expressed as numbers and percentages and were compared by χ2 or Fisher exact test with continuity correction. P < 0.05 was considered to be of a statistical significance. RevMan software version 5.4.1 was applied for assessing the heterogeneity of the literature and drawing a forest plot.
Results
Study material
The recruited articles included retrospective studies (n = 20) [5, 10, 13, 15–17, 20–23, 25, 26, 28, 30, 32, 36, 37, 39, 41, 42], case reports (n = 13) [3, 4, 8, 9, 12, 14, 18, 24, 27, 29, 31, 33, 35], case series (n = 2) [11, 34], medical imaging (n = 1) [19], pilot projects (n = 1) [40], and a letter to the editor (n = 1) [38]. A forest plot is shown in Figure 2.
Patient information
In total 401 patients with Alagille syndrome with pulmonary artery pathologies were included. The gender of 117 patients was described with 77 (65.8%) male and 40 (34.2%) female patients (χ2 = 11.9, p < 0.001). There were 386 (96.3%) pediatrics, 11 (2.8%) adults and 4 (1.0%) fetuses. Their ages were 4.1 ±3.2 (range: 0–12.4; median: 3.5) years (n = 34), 38 ±13.9 (range: 21–54; median: 40) years (n = 5) and 29.4 ±6.2 (range: 21–34; median: 31.3) weeks of gestation (n = 4) for pediatrics, adults and fetuses, respectively.
The age at diagnosis of Alagille syndrome and (or) pulmonary artery pathology was 1.4 ±3.1 (range: 0–12.5; median: 0.17) years (n = 17) [4, 5, 9–12, 27, 29, 33, 35, 38] for pediatrics and adults, and at 28.57 weeks of gestation for one fetus [8] and after birth for 3 fetuses [36]. JAG1 gene mutation was detected in 129 (32.2%) patients [3, 5, 8, 25, 34, 36, 39] as well as the mother of one of the fetuses [8].
Except for the most common presenting symptoms of Alagille syndrome including jaundice and pruritus, other symptoms reported were fetal intrauterine growth retardation (n = 17) [8, 30, 36], raised spiny lesions (n = 1) [18], dyspnea and chest discomfort (n = 1) [19] and dyspnea and headache (n = 1) [35]. A systolic murmur and a systolic and diastolic murmur were heard in 12 [4, 8, 9, 11, 12, 27, 31, 33] and 1 patient [35], respectively. The 103 associated conditions of 82 patients are shown in Table I.
Table I
A hundred and three associated conditions of 82 patients
| Associated condition | n (%) |
|---|---|
| Tetralogy of Fallot [5, 11, 16, 22, 38] | 47 (45.6) |
| Abdominal aorta narrowing [31] | 11 (10.7) |
| Atrial septal defect [11, 14, 20, 40] | 6 (5.8) |
| Inter-renal aortic stenosis (renovascular hypertension) [10, 34] | 6 (5.8) |
| Ventricular septal defect [8, 16, 30, 32, 40] | 5 (4.9) |
| Idiopathic descending aorta constriction [40] | 3 (2.9) |
| Aortic stenosis [14, 20] | 2 (1.9) |
| Patent foramen ovale [32, 33] | 2 (1.9) |
| s/p hepatic portocholecystostomy [13] | 2 (1.9) |
| s/p left pulmonary artery stenting [32] | 2 (1.9) |
| Abnormal pulmonary outflow tract [11] | 1 (1.0) |
| Calcified atheroma plaques and poststenotic dilation of the pulmonary arteries [19] | 1 (1.0) |
| Coarctation of the aorta [20] | 1 (1.0) |
| Granuloma annulare [40] | 1 (1.0) |
| Hypoplastic left heart syndrome [40] | 1 (1.0) |
| Multiple dermal cysts [12] | 1 (1.0) |
| s/p surgical pulmonary valvotomy [35] | 1 (1.0) |
| s/p aortic valvotomy + right ventricular outflow tract conduit insertion [38] | 1 (1.0) |
| s/p atrial septal defect closure [35] | 1 (1.0) |
| s/p liver transplantation [37] | 1 (1.0) |
| s/p modified Taussig-Bing shunt [33] | 1 (1.0) |
| s/p patent ductus arteriosus closure [35] | 1 (1.0) |
| s/p right ventricular outflow tract conduit replacement [38] | 1 (1.0) |
| s/p staged palliation of tetralogy of Fallot [24] | 1 (1.0) |
| s/p unifocalization of major aortopulmonary collateral arteries, aortopulmonary window takedown and placement of a 4-mm central shunt [24] | 1 (1.0) |
| s/p ventriculoperitoneal shunt [11] | 1 (1.0) |
| Ventricular septal defect with pulmonary atresia [16] | 1 (1.0) |
Pulmonary artery stenosis
The laterality of pulmonary stenosis was stated for 35 patients: it was bilateral in 31 (88.6%) [3, 4, 22, 24, 26, 27, 29, 33, 35, 38], on the left in 3 (8.6%) [9, 11, 37] and on the right in 1 (2.9%) patient [37]. The severity of pulmonary artery stenosis was reported for 30 pulmonary arteries of 28 patients. It was severe/critical in 21 (69.0%) [8, 28, 29, 33, 35], moderate in 5 (17.2%) [11, 24, 27, 34, 35] and mild in 4 (13.8%) pulmonary arteries [12, 14, 24, 38].
Isolated pulmonary stenosis was found in 302 (75.3%) cases [3, 4, 5, 8, 9, 11–37, 39–42], while pulmonary stenosis with complex cardiac or vascular abnormalities was found in 86 (21.4%) cases [5, 10, 11, 16, 22, 25, 33, 36, 38, 42]. Of them, tetralogy of Fallot was present in 71 (82.6%, 71/86) patients. In one of the reports referring to 29 cases of tetralogy of Fallot [22], the pulmonary arterial status was not indicated. In the remaining 42 patients, 28 (66.7%) had pulmonary stenosis [5, 11, 16, 25, 42], 13 (31.0%) had pulmonary atresia [16, 25, 42] and 1 (2.4%) patient had absence of the pulmonary artery [25]. In addition, pulmonary hypoplasia was present in 10 (2.5%, 10/401) patients [3, 8, 11, 30, 31, 33, 37].
Pulmonary stenosis with simple congenital or acquired heart disease was found in 13 (3.2%) cases, including atrial septal defect (n = 4) [11, 20], ventricular septal defect (n = 3) [5, 16, 30], aortic stenosis (n = 2) [5, 20], hypertrophic cardiomyopathy (n = 1) [5] and coarctation of the aorta (n = 1) [20].
The level of pulmonary stenosis was described for 259 patients, including peripheral in 231 (89.2%) [3–5, 9–11, 13, 15–19, 21–32, 34–38, 40], valvular in 23 (8.9%) [5, 11, 16, 25], main and peripheral in 2 (0.8%) [11, 33], valvular and infundibular in 1 (0.4%) [11], supravalvular and peripheral in 1 (0.4%) [5], and valvular and peripheral in 1 (0.4%) patient [5]. Preoperative systolic pulmonary artery pressure was 61.7 ±22.2 (range: 29–86; median: 72) mm Hg (n = 7) [3, 9, 24, 40].
There were no differences found between preoperative and postoperative right ventricular pressures (68.6 ±26.4 mm Hg vs. 73.0 ±27.0 mm Hg, p = 0.758) or between preoperative and postoperative right ventricular pressure/left ventricular pressure ratios (0.8 ±0.3 vs. 0.6 ±0.3, p = 0.162). A comparison of postoperative right ventricular pressure/left ventricular pressure ratios between interventionally treated patients with no residual pulmonary artery stenosis (0.59 ±0.18), interventionally treated patients with residual pulmonary artery stenosis (0.94 ±0.23) and surgically treated patients (0.37 ±0.01) did not reveal any significant differences even though the interventionally treated patients with residual pulmonary artery stenosis showed the highest ratio (Figure 3).
Treatment and outcomes
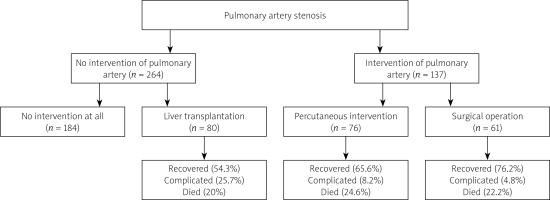
Pulmonary artery stenosis was not intervened for 264 patients and 80 (30.3%) of them received liver transplantation [12–14, 20, 21, 28, 30, 39, 41]. The outcomes of these 80 patients were not indicated for 10 of them. Of the remaining 70 patients, 38 (54.3%) recovered, 18 (25.7%) were complicated and 14 (20%) died.
In the remaining 137 patients, 76 patients underwent 109 percutaneous interventions: 103 (94.5%) were primary interventions including 32 (31.1%) balloon angioplasty (BA) [5, 9, 29, 30, 37, 38, 42], 3 (2.9%) cutting BA [42], 1 (1.0%) high pressure BA [17], 18 (17.5%) stenting [3, 9, 11, 15, 22, 33, 35, 42] (1 of them underwent subsequent stent dilation [33]), 1 (1.0%) BA + stenting [37], 2 (1.9%) percutaneous valvuloplasty [5] and 46 (44.7%) cardiovascular interventions [25]; and 6 (5.5%) were reinterventions including the subsequent stent dilation. Other operations that were performed in the patients of this group were orthotopic liver transplantation in 3 patients [30], right ventricular outflow track reconstruction and unifocalization of major aortopulmonary collateral arteries in 1 patient [5], and living donor liver transplantation with later endovascular stent for portal vein stenosis in 1 patient [29].
Surgical operation for pulmonary artery stenosis was performed in 61 patients including 57 primary operations and 4 reoperations (Table II).
Table II
Surgical operations for pulmonary artery stenosis
| Surgical operation | n (%) |
|---|---|
| Primary operation: | 57 (93.4) |
| Patch augmentation of the left ventricular outflow tract or pulmonary artery [11, 23] | 13 (22.8) |
| Pulmonary valvuloplasty [11] | 2 (3.5) |
| Revision of bilateral branch pulmonary arteries [24] | 1 (1.8) |
| Unspecified surgical repair [22] | 41 (71.9) |
| Re-operation: | 4 (6.6) |
| Repair for shunt dehiscence at the aortic site [24] | 3 (75) |
| Later patch augmentation of the left pulmonary artery [11] | 1 (25) |
The outcomes of the remaining 61 interventionally and 63 surgically treated patients from 21 reports (in which patient outcomes were given) are shown in Table III. There were no intergroup differences in terms of recovery, reintervention and mortality rates. The complications of the interventional group patients were 3 minor complications (2 small contained vascular tears and 1 stent embolization not requiring surgery) and 1 major complication due to hemorrhage from esophageal varices which caused patient death in 1 report [42] and right pulmonary artery stent recoil that required a stent-in-stent treatment [35]. The complications of the surgical group were residual proximal branch pulmonary artery stenosis warranting a balloon angioplasty [23], shunt dehiscence occurred 3 times which were surgically repaired but finally died of septic shock [24] and another patient was complicated and died of pulmonary hypertensive crisis [8].
Table III
A comparison of outcomes between interventional and surgical group patients
| Group | Recovered | Complicated | Recurrence | Reintervention | Died |
|---|---|---|---|---|---|
| Intervention (n = 61) | 40 (65.6) | 5 (8.2)* | 2 (3.3)** | 3 (4.9) | 15 (24.6) |
| Surgery (n = 63) | 48 (76.2) | 3 (4.8)*** | 2 (3.2) | 14 (22.2) | |
| χ2 | 1.7 | 0.6 | 2.1 | 0.2 | 0.1 |
| P-value | 0.237 | 0.488 | 0.240 | 0.677 | 0.833 |
Discussion
Pulmonary artery stenosis in the context of congenital heart defects, such as tetralogy of Fallot, truncus arteriosus, transposition of the great arteries, single ventricle heart disease, pulmonary atresia, or congenital rubella may be conjointly attributed to the development of pulmonary artery stenosis in Alagille syndrome [15]. The diagnosis of Alagille syndrome relies on clinical symptoms, systolic murmur best heard at the left upper sternal border and medical imaging, as well as mutations of JAG1. Alagille syndrome is reported to be caused by mutations of JAG1 in 94% of patients [43]. In this cohort of Alagille syndrome with pulmonary artery pathologies, the mutations of JAG1 accounted for 32.2%.
As it has been proved by Ahn et al. [5], pulmonary artery stenosis was the most common heart defect of Alagille syndrome accounting for 97.2% with 71.4% being peripheral pulmonary artery stenosis, followed by pulmonary stenosis at the valvular (14.3%), supravalvular pulmonary with peripheral (8.6%), and valvular with peripheral levels (5.7%).
The treatment of peripheral pulmonary artery stenosis includes surgery, BA and pulmonary artery stenting. A procedure leading to an increase in the pulmonary artery diameter by ≥ 50% or a decrease in the right ventricular to left ventricular pressure ratio by ≥ 20% is regarded as a success. Stenting was associated with a favorable outcome but without statistically surpassing that of open surgery [44]. In the present study, although the right ventricular pressures did not show a significant reduction after the procedure, the right ventricular to left ventricular pressure ratio was reduced by 25%, indicating the remarkable therapeutic effects of interventional and surgical management.
Surgical repair of the peripheral pulmonary artery is technically challenging. The techniques included reconstruction of the lobar branch pulmonary artery along with augmentation of the central and main pulmonary arteries, which resulted in 57% reduction in the right ventricle to aortic pressure ratios, and homograft patch augmentation for long-segment stenosis and surgical ostioplasty for localized ostial disease. However, it was stated that surgical repair of the peripheral pulmonary artery was disappointing with a 50–60% restenosis rate at 5 years [45]. Nevertheless, supravalvular pulmonary stenosis or bifurcation stenosis of the branch pulmonary arteries are curable to surgical repair [45]. Surgical repair of the peripheral pulmonary artery can be performed concurrently with the repair of intracardiac lesions, or simultaneous surgical repair of a pulmonary arterial lesion with palliative systemic-to-pulmonary artery shunt may also be performed [45].
Indications for liver transplantation in patients with Alagille syndrome were refractory pruritus, decompensated cirrhosis, recurrent upper gastrointestinal bleeding, and hepatopulmonary syndrome [21]. Ovaert et al. [28] described 17 patients with Alagille syndrome undergoing liver transplantation at a mean age of 3.5 years. All patients had pulmonary artery stenosis but none was symptomatic. Razavi et al. [32] pointed out that patients with Alagille syndrome and pulmonary artery lesions should attempt transcatheter stenting for pulmonary artery stenosis in patients following liver transplantation when they had failure of increase in the cardiac index by 40% or had a right ventricular pressure over half systemic pressure.
Associated intracardiac congenital heart disease, especially that found by fetal ultrasonography, and combined severe liver disease and severe heart disease were adverse predictive factors of poor survival [5]. Emerick et al. [16] reported the outcome of patients with Alagille syndrome was associated with increased mortality when there was congenital heart disease present, with an estimated survival of 40% at 20 years, compared to 80% at 20 years in its absence. Survival of Alagille patients with tetralogy of Fallot or pulmonary atresia with the ventricular septal defect was 66% and 25%, respectively, considerably poorer than the reported survival at 10 years of 89% and 58%, respectively in patients without Alagille syndrome. The increased mortality of intervention and surgery could be attributed to: the associated cardiopulmonary malformations (tetralogy of Fallot with or without major aortopulmonary collaterals [22, 25], truncus arteriosus [23], supravalvar aortic stenosis with a gradient of 70 mm Hg [23], sinus of Valsalva aneurysm [25], Williams’ syndrome [23] and severe peripheral pulmonary artery stenosis [22, 23]), and a complex surgical procedure with a total bilirubin level of > 15 mg/dl [5].
Patient information of many reports was incomplete, and patient information in the large sample cohorts was often missing. For instance, postoperative pulmonary artery pressures were not given by all reports and a comparison of pulmonary artery pressures between pre-operation and post-operation became impossible. This aspect constituted the main drawback of this study.
Conclusions
In patients with Alagille syndrome, pulmonary artery pathologies, especially a severe type of pulmonary artery stenosis warrant surgical or interventional treatment. After the procedures, the right ventricular to left ventricular pressure ratio was reduced by 25%. There were no intergroup differences in terms of recovery, reintervention and mortality rates between interventionally and surgically treated patients.