Introduction
Turner syndrome (TS) is a genetic disorder in which there are structural or quantitative aberrations of one of the X chromosomes in women [1]. It is characterized by hypogonadism, short stature, typical phenotypic features, frequent occurrence of congenital heart, kidneys, vision and hearing defects and other associated diseases [1]. The latter include autoimmune diseases, thyroid disorders or diabetes mellitus. Vascular malformations in the gastrointestinal tract (GI) belong to rare and relatively hard to diagnose defects in these patients. The first case was described by Lisser in 1947 and next by other authors [2]. The incidence of GI vessels malformations in TS patients is estimated for 7% [3]. They can be asymptomatic, but also cause latent GI bleedings, leading to iron deficiency anemia, and in single cases can cause acute and recurrent GI bleeding [3, 4]. The aim of this study was to present the case of a TS patient with such a problem and to show the current diagnostic tools of detection of vascular malformations in the GI tract.
Case report
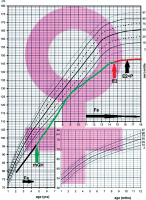
A 12-year-old patient with TS, short stature and heart defects –aortic stenosis and partial anomalous pulmonary venous drainage. The Turner syndrome was diagnosed prenatally. The karyotype 45, X was additionally confirmed after the birth in peripheral lymphocytes. In preschool period she was treated with iron preparations because of anemia of unknown origin (Fig. 1). From 4th until 15th year of life she was treated with rhGH (0.35 mg/kg/week). She improved growth velocity, but after 12 years she inhibited growth (Fig. 1). Because of that she was investigated. Thyroid function was normal, celiac disease was excluded, but severe microcytic anemia was found (Table I).
Table I
Elective CBC parameters correlated with Iron serum concentration and faecal occult blood test in the course of obser- vation of the patient
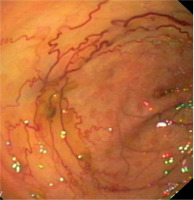
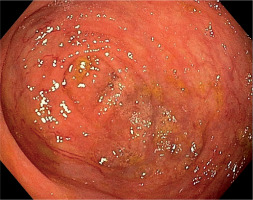
Initially, she was diagnosed in the hematological ward, where the most common hematological diseases were excluded. She was treated with iron preparations in therapeutic doses, but the effect was not satisfactory (Table I). Laboratory tests revealed significant red blood cell anisocytosis with a predominance of microcytes, poikilocytosis, hypochromia and inconsiderable platelet anisocytosis, as well as low iron concentration with a normal absorption and a double positive occult blood test. She continued the diagnostics in the Department of Pediatric Gastroenterology and Nutrition. This time the patient didn’t report any complaints. Her bowel movements were normal. Abdominal ultrasound, gastroscopy and scintigraphy towards Meckel’s diverticulum didn’t show any abnormalities. Colonoscopy and capsule endoscopy were performed. Widened venous vessels, just below the macroscopically unchanged mucosa, were visualized along the entire length of the large intestine and in the terminal ileum (Fig. 2). Argon coagulation of the lesions was considered, however, due to the large number of vascular malformations and the lack of features of active bleeding, it was abandoned. Supplementation with iron preparations was recommended and she needed the permanent treatment for three years with therapeutic doses of iron. At the age of 14.5 years she terminated GH treatment and due to increasing gonadotropins level, the patient started sex hormone replacement therapy (initially only estrogen (17β-estradiol beginning with 0,5 mg), and after 1,5 year complete replacement with estrogen (estradiol 1 mg) and a progestagen (norethisterone acetate 1 mg). Since then, for the 3 years of observation, no GI bleeding occurred. In control colonoscopy, there was a significant decrease in the number of vascular malformations in the large intestine (Fig. 3).
Discussion
This case supports the idea that in TS patients the differential diagnosis of iron deficiency anemia should include chronic bleeding due to vascular defects in the GI tract. Inflammatory bowel disease, polyps, peptic ulcer, celiac disease, and injuries should be excluded [3, 5-7]. Apart from laboratory testing, initial diagnostics should include endoscopic examination of the upper and lower gastrointestinal tract [7], however, these methods are only effective when vascular anomalies are located in duodenum or large intestine [6]. The malformations may have different locations, but they are mostly present in the small intestine [3, 5-9]. Diagnostic difficulty is the fact that although all layers of the intestinal wall may be involved, the lesions are mainly located in the serosa [5, 6, 8]. Currently, the capsule endoscopy is considered as the best method in the evaluation of changes in the small intestine [5, 6, 9]. This method is a useful and effective tool in case of suspected GI bleeding of unclear origin [5]. The accurate source of bleeding could be identified in approximately half of the patients undergoing this test [10].
In the majority of documented cases in the literature, bleeding episodes most often occurred during the first two decades of life [3, 6, 8, 9] and their frequency tend to spontaneously decline with the age [3, 6, 8]. There are theories that this may be dependent on the increase of sex hormone levels during spontaneous or artificially induced puberty [3]. It is believed that estrogens protect against formation of vascular malformation in the intestinal wall [3, 7], prevent bleeding and reduce bleeding from existing lesions. Estrogens cause an increased coagulation with stasis in microcirculation, have a trophic effect on the intestinal mucosa and the direct effects stabilizing the endothelial lining of the abnormal blood vessels [6, 8]. Estrogens were also proven to influence the production of hepatic coagulation factors and thereby they could reduce bleeding time [3]. There were described several cases of patients with TS and GI bleeding, in whom improvement was observed after the implementation of estrogen therapy [3]. In our patient after the start of estrogen supplementation no GI bleeding was observed within 2 years of follow-up. Moreover, the control colonoscopy showed lesser number of the malformations in the large intestine. We presume that it may be the beneficial effect of estrogen substitution. However, the mechanism of female sex hormone influence is difficult to interpret because small number of reported patients [7, 8]. The rarity of vascular malformations in GI tract in TS patients make difficult the prospective studies on large groups of patients, and to draw reliable and unambiguous conclusions [3].
Conclusions
The case of our patient supports the idea that if a patient with TS develops iron deficiency anemia, especially with recurrent or overt GI bleeding, the vascular malformations of GI tract should always be considered in the differential diagnosis. Apart from gastroscopy and colonoscopy, capsule endoscopy is very useful to evaluate the small intestine. Female sex hormones supplementation may significantly reduce the risk of bleeding. However, further research on larger groups of patients is needed to confirm this suggestion and possibly use of sex hormone replacement therapy as a targeted treatment for vascular malformations in TS.

 POLSKI
POLSKI