Introduction
Pulmonary resection is the most common treatment for stage I to stage III lung cancer [1]. Video-assisted thoracoscopic surgery (VATS) is a minimally invasive technique, which is widely used worldwide. Compared to thoracotomy, VATS offers more advantages such as faster postoperative recovery, less postoperative pain, shorter length of hospital stay, greater preservation of pulmonary function, and lower perioperative morbidity [2]. Although VATS has lots of advantages, postoperative complications can still occur. Extensive subcutaneous emphysema is one of the serious complications after pulmonary resection, especially in the setting of delayed treatment. However, few studies have reported whether extensive subcutaneous emphysema can occur after VATS.
Extensive subcutaneous emphysema is defined as the entry of air into the subcutaneous space of the chest wall, followed by spread to soft tissues of the upper chest, shoulder, abdomen, neck, head, face, and eyelids, causing distress to the patient, in addition to pneumothorax, pneumomediastinum, pneumoperitoneum, temporary visual impairment, or even respiratory and cardiovascular collapse [3–5]. The reported incidence of extensive subcutaneous emphysema after an elective thoracic procedure is approximately 1.96% (21/1069) [3]. Extensive subcutaneous emphysema can not only lead to physical discomfort and cosmetic problems, but can also significantly prolong the length of hospital stay, and increase the medical costs and burden of patients. More importantly, a delay in treatment could lead to respiratory arrest and subsequent death in patients with extensive subcutaneous emphysema, as reported in previous studies [6, 7]. Therefore, it is important to perform an in-depth exploration of the risk factors for extensive subcutaneous emphysema after pulmonary resection through VATS.
To the best of our knowledge, there is a paucity of data on the risk factors for extensive subcutaneous emphysema after VATS pulmonary resection. The majority of the previous studies were case reports focusing on the most accurate and efficacious diagnosis and treatment methods for extensive subcutaneous emphysema [8–11]. Cerlofio et al. conducted a cohort study and found that after previous thoracotomy, air leak and preoperative forced expiratory volume of air in 1 s (FEV1) less than 50% were related to the occurrence of subcutaneous emphysema [12]. In their study, the included patients underwent either VATS or thoracotomy; lobectomy and segmentectomy were performed through thoracotomy, while only wedge resection was performed through VATS. However, since 2002, the VATS technique has been implemented in large thoracic surgical procedures, including lobectomy, segmentectomy, sleeve resection, and even pneumonectomy [13]. Since the indications for VATS have changed greatly over the last decade, it is necessary to discuss the risk factors for extensive subcutaneous emphysema following VATS pulmonary resection.
Therefore, we designed a matched case-control study to explore the risk factors for extensive subcutaneous emphysema after pulmonary resection by VATS. Univariate and multivariate logistic regression analyses were performed to select the risk factors and calculate the odds ratio (OR) to clarify the relationship between the risk factors and extensive subcutaneous emphysema. It is hoped that the findings of this study can help in preventing postoperative extensive subcutaneous emphysema and reduce its incidence, thus improving the postoperative clinical outcome and offering a better patient experience.
Aim
The primary objective of this study was to identify the risk factors for extensive subcutaneous emphysema after pulmonary resection through VATS, and the secondary objective was to analyze the incidence rate and occurrence time of extensive subcutaneous emphysema.
Material and methods
Design
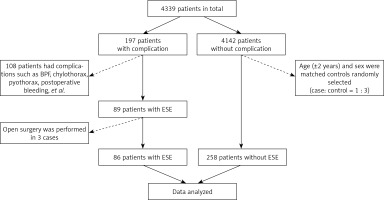
The present study is a retrospective matched case-control investigation that evaluated patients admitted to the thoracic surgery department of Shanghai Chest Hospital, a tertiary-referral and university-affiliated hospital, between October 2018 and October 2020. A total of 344 patients were enrolled in the study, including 86 cases and 258 matched controls.
Participants
The inclusion criteria were (I) those who underwent pulmonary resection through VATS surgery; (II) patients who were admitted to this hospital between October 2018 and October 2020; (III) age ≥ 18 years. The exclusion criteria were (I) those who underwent thoracotomy; (II) those who did not receive surgical treatment; (III) and incomplete medical records. The cases were patients who were admitted in the study period and experienced extensive subcutaneous emphysema after pulmonary resection through VATS surgery. Diagnosis of extensive subcutaneous emphysema was confirmed by the detection of air inside the soft tissues by chest X-ray and/or thoracic computed tomography as well as by clinical symptoms. The severity of subcutaneous emphysema has been classified by Aghajanzadeh et al., whereby grade I indicates the base of the neck, grade II indicates all of the neck area, grade III indicates the subpectoralis major area, grade IV indicates the chest wall and all of the neck area, and grade V indicates the chest wall, neck, orbit, scalp, abdominal wall, upper limbs, and scrotum [14]. Patients who had subcutaneous emphysema of grade 4 or 5, which was radiologically apparent, were included in the case group. For the control group, patients were selected from the remaining population and frequency matched in a ratio of 3 : 1 to the cases based on the age (±2 years) and sex in the same ward and over the same period.
Data collection
Data regarding the basic clinical characteristics and operation-related characteristics were extracted from the electronic hospital database. Basic clinical characteristics included sex, age, body mass index (BMI), pulmonary function, comorbidities, history of malignancy, history of lung surgery, and preoperative albumin. Meanwhile, operation-related characteristics were also documented, such as the time of operation, intraoperative blood loss, surgical approach, pleural adhesions, and histology. Among the variables, pleural adhesions were divided into three groups: no, minimal, and extensive pleural adhesions. Minimal pleural adhesions were defined as those requiring adhesiolysis within 30 min, while extensive pleural adhesions required adhesiolysis for 30 min or longer [15].
Ethical consideration
This study was approved by the Medical Ethics Committee of Shanghai Chest Hospital (approval number: IS21125). The requirement for informed consent was waived by the ethics committee.
Statistical analysis
The demographic and perioperative variables were compared between the case and control groups. The χ2 test or, if necessary, Fisher’s exact test was used to compare the categorical variables, while the Wilcoxon signed ranks test was used to compare the continuous variables. All variables that displayed a significant univariate association (p < 0.2) were entered into a multivariate logistic regression model. The relationship between the risk factors and extensive subcutaneous emphysema was quantified using the OR and 95% confidence intervals (CI). Statistical analyses were performed using SPSS (version 23.0, Inc., Chicago, IL, USA).
Results
Sample characteristics
From October 2018 to October 2020, 4339 continuous patients were admitted to the thoracic surgical ward of Shanghai Chest Hospital for treatment. Among them, postoperative complications occurred in 197 patients after pulmonary resection, while there were no complications in 4142 patients. A total of 108 patients were excluded from this study as they experienced other postoperative complications, such as bronchopleural fistula, chylothorax, pyothorax, and postoperative bleeding. Additionally, 3 patients with extensive subcutaneous emphysema were eliminated from the case group as they had undergone open surgery. Age (±2 years) and sex were used as the matching factors to screen all controls from the same ward and over the same period. Each patient in the case group was matched with 3 control patients. Finally, the data of 344 patients were analyzed in this study (86 of 89 cases and 258 of 4142 controls). A flowchart of the study population is presented in Figure 1.
Univariate analysis
In this study, 86 patients were included in the case group and 258 patients in the matched control group. Basic clinical characteristics of the cases and matched controls are shown in Table I. The mean ages of the patients in the case and matched control groups were 64.92 and 64.70 years, respectively. About 77.9% of the patients in both groups were men, while 22.1% of the patients were women. Most patients (41.9%) in both groups had hypertension. The occurrence rate of diabetes mellitus was 12.8% in the case group and 16.7% in the matched control group. The case group demonstrated a lower average body mass index (BMI) (23.32 vs. 24.29 kg/m2, p = 0.009), lower average FEV1 value (86.2 vs. 94.05, p = 0.006), lower average FEV1/forced volume vital capacity (FVC) ratio (94.5 vs. 100.59, p = 0.005), and a lower average diffusing capacity of the lungs for carbon monoxide (DLCO) (89.08 vs. 95.55, p = 0.035). There were no significant differences in sex, age, FVC, comorbidities, history of malignancy, history of lung surgery, and preoperative albumin between the two groups (p > 0.05).
Table I
Basic clinical characteristics of cases and controls
| Variables | Cases (n (%), mean ± SD) | Controls (n (%), mean ± SD) | P-value |
|---|---|---|---|
| Sex: | 1.000a | ||
| Male | 67 (77.9) | 201 (77.9) | |
| Female | 19 (22.1) | 57 (22.1) | |
| Age | 64.92 ±9.27 (35–83) | 64.70 ±9.03 (33–81) | 0.632b |
| BMI | 23.32 ±3.69 (13.8–33.7) | 24.29 ±2.93 (16–37.3) | 0.009b |
| Pulmonary function: | |||
| FVC | 90.64 ±17.1 (55.1–173.2) | 92.12 ±14.39 (50–125.6) | 0.364b |
| FEV1 | 86.2 ±18.49 (41.6–124.4) | 94.05 ±17.3 (40.6–133.3) | 0.006b |
| FEV1/FVC | 94.5 ±12.95 (51.8–117.9) | 100.59 ±9.62 (61.1–129.9) | 0.005b |
| DLCO | 89.08 ±25.95 (46.6–206.6) | 95.55 ±19.39 (27.4–148.4) | 0.035b |
| Comorbidities: | |||
| Hypertension | 36 (41.9) | 108 (41.9) | 1.000a |
| Diabetes mellitus | 11 (12.8) | 43 (16.7) | 0.494a |
| Heart disease | 7 (8.1) | 24 (9.3) | 0.831a |
| History of malignancy | 8 (9.3) | 21 (8.1) | 0.823a |
| History of lung surgery | 7 (8.1) | 9 (3.5) | 0.134a |
| Preoperative albumin | 41.41 ±3.12 (29–47) | 41.74 ±3.35 (26–50) | 0.785b |
Table II shows the operation-related characteristics of the cases and controls. The mean time of the operation in the case and matched control groups was 99.22 and 88.09 min, respectively. The average intraoperative blood loss was greater in the case group than in the matched controls (78.95 vs. 66.34 ml, p = 0.039). Lobectomy was the most common type of resection, accounting for 65.1% of resections in the case group and 53.5% in the matched control group. About 88.4% of the patients in the case group and 91.9% in the matched control groups had malignancy (p = 0.328). In the case population, there were more patients with minimal (9.3% vs. 5.4%) to extensive (25.6% vs. 5.4%) pleural adhesions than in the matched control group (p < 0.001). There were no significant differences in the histology between the two groups (p > 0.05).
Table II
Operation-related characteristics of cases and controls
| Variables | Cases (n (%), mean ± SD) | Controls (n (%), mean ± SD) | P-value |
|---|---|---|---|
| Time of operation | 99.22 ±30.65 (45–180) | 88.09 ±33.52 (30–240) | 0.004b |
| Intraoperative blood loss | 78.95 ±35.94 (10–200) | 66.34 ±34.79 (10–200) | 0.039b |
| Surgical approach: | 0.013c | ||
| Wedge resection | 9 (10.5) | 68 (26.4) | |
| Segmentectomy | 17 (19.8) | 47 (18.2) | |
| Lobectomy | 56 (65.1) | 138 (53.5) | |
| Sleeve lobectomy | 4 (4.7) | 5 (1.9) | |
| Pleural adhesion: | 0.000a | ||
| None | 56 (65.1) | 230 (89.1) | |
| Minimal | 8 (9.3) | 14 (5.4) | |
| Extensive | 22 (25.6) | 14 (5.4) | |
| Histology: | 0.328a | ||
| Benign tumor | 10 (11.6) | 21 (8.1) | |
| Malignancy | 76 (88.4) | 237 (91.9) |
Logistic regression analysis
In univariate analysis, nine factors were found to be associated with the incidence of extensive subcutaneous emphysema. Further analysis using the multivariate logistic regression model is presented in Table III. Among the basic clinical characteristics, FEV1/FVC was found to be a protective factor based on multivariate logistic regression (OR = 0.959, 95% CI: 0.927–0.992, p = 0.015). In terms of the operation-related characteristics, the type of resection and pleural adhesions were significantly associated with the incidence of extensive subcutaneous emphysema (p < 0.05). The results also showed that patients undergoing segmentectomy (OR = 3.130, 95% CI: 1.055–9.283, p = 0.040) and lobectomy (OR = 4.487, 95% CI: 1.704–11.812, p = 0.002) had higher odds of developing extensive subcutaneous emphysema than those undergoing wedge resection. Similarly, patients with extensive pleural adhesions had a 4.514-fold higher risk of extensive subcutaneous emphysema than those without pleural adhesions (OR = 4.514, 95% CI: 1.763–11.556, p = 0.002).
Table III
Risk factors of extensive subcutaneous emphysema
Discussion
Nowadays, the VATS technique is widely used for the diagnosis of mediastinal, lung, and pleural disease and adopted for anatomical lung resections, as well as for large pulmonary resection procedures that meet surgical qualifications, such as sleeve lobectomy and pneumonectomy [13, 16]. Extensive subcutaneous emphysema is one of the postoperative complications of VATS lung resection, causing significant anxiety and prolonging hospital stay. It causes symptoms such as swelling, breathlessness, wheezing, distension, pain, tenderness, sore throat, and difficulty in swallowing [17]. Furthermore, the sudden appearance of swelling can increase the fear and anxiety among patients and their families, and can trigger conflicts between the patients and medical staff. There are two mechanisms of subcutaneous emphysema. In one, air from a pneumothorax may enter directly into the chest wall and subcutaneous tissue when the parietal pleura is pierced [18]. In the other, rupture of the alveoli at the bottom can introduce air into the perivascular adventitia, which tends to dissect proximally within the bronchovascular sheath towards the mediastinum, as described by Macklin in 1939 [19]. When air leakage is greater than reabsorption, progressive accumulation occurs in the various tissue planes, leading to the development of subcutaneous emphysema. However, the detailed risk factors of extensive subcutaneous emphysema after pulmonary resection through VATS needed further elucidation.
A number of factors account for extensive subcutaneous emphysema, including trauma, pneumothorax, malignancy, and infection, or it can occur as a complication of surgical procedures [8, 17, 20, 21] (Figure 2). Kim et al. stated that old age and large pneumothorax were inherent risk factors for extensive subcutaneous emphysema after closed-tube thoracostomy [22]. In addition, poor pulmonary function, presence of pleural adhesions, and high-dose steroid use for more than 1 month were risk factors for postoperative subcutaneous emphysema according to a study by Singhal et al. [23]. However, these previous studies focused on extensive subcutaneous emphysema in patients undergoing open thoracotomy [8, 24, 25]. Little attention has been paid to the risk factors for extensive subcutaneous emphysema after pulmonary resection through VATS. Therefore, this matched case-control study was designed to explore the potential risk factors for extensive subcutaneous emphysema in patients receiving pulmonary resection via VATS, which might provide valuable clinical guidance and support clinical decision-making.
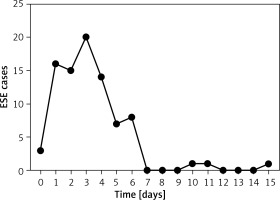
In this present study, the incidence of extensive subcutaneous emphysema after pulmonary resection was 2.05% (89/4339), which is similar to the results of Boulemden et al. [3]. In addition, about 75.58% (65/86) of the cases of extensive subcutaneous emphysema occurred within 1 to 4 days after surgery. The finding might provide an important reference for the early identification of extensive subcutaneous emphysema after pulmonary resection by VATS in clinical practice. Kim et al. and Boulemden et al. also observed that extensive subcutaneous emphysema occurred within 1 and 3 days postoperatively [3, 22], respectively, which was consistent with our results. However, there was a difference in the inclusion criteria between these two studies and the present study. The inclusion criteria of former studies included more surgical techniques such as thoracostomy, open lung resection, lung volume reduction surgery, and VATS. Notably, the occurrence time of extensive subcutaneous emphysema in the case of VATS lung resection has been relatively poorly researched. Further research and deeper understanding of the occurrence time of extensive subcutaneous emphysema are of considerable importance for early identification and diagnosis in clinical practice.
In order to further identify the risk factors for extensive subcutaneous emphysema, a variety of possible parameters including BMI, FEV1, FEV1/FVC, DLCO, intraoperative blood loss, time of operation, and history of lung surgery were examined in this study. These parameters were found to be statistically significant in the univariate analysis; however, these factors were not identified as independent risk factors in the multivariate analysis. Logistic regression is an effective and powerful method to analyze the influence of a set of independent variables on binary results by quantifying the unique contribution of each independent variable [26]. The results of multivariate logistic regression showed that the type of resection and pleural adhesions were independent risk factors for extensive subcutaneous emphysema after pulmonary resection through VATS. Pischik et al. reported that lobectomy and pleural adhesions were independent risk factors for prolonged air leak after pulmonary resection, which is consistent with the findings of the present study [27]. The occurrence of prolonged air leak increases the risk of subcutaneous emphysema, which explains why some of the risk factors for prolonged air leak and extensive subcutaneous emphysema are similar. Furthermore, approximately 83.72% (72/86) of the patients in the present study demonstrated various degrees of air leak postoperatively. Extensive pleural adhesions were defined as those requiring adhesiolysis for 30 min or longer [15]. The impact of pleural adhesions on subcutaneous emphysema is not surprising given that the massive pleural adhesions worsen visualization during the operation and impair the control of surgeons during dissection, which may result in pulmonary parenchyma injury and air leak [27].
The strength of this study is that we identified independent risk factors for extensive subcutaneous emphysema by logistic regression analysis in addition to analyzing its occurrence time by the trend shown in a line chart. The findings of this study are expected to raise awareness among nurses and surgeons regarding the prevention and management of extensive subcutaneous emphysema in patients with risk factors. However, there are some potential limitations of this study. First of all, the retrospective data may be incomplete or inaccurate; for example, the data on the patients’ preoperative comorbidities may be inaccurate due to the subjective information given by the patients or their families. Secondly, the data were collected from a single center; although the research findings were partially congruent between our study and the studies conducted in other countries, differences in the culture and the level of health care services between countries may affect the generalizability of this study. Therefore, prospective multicenter studies are required to acquire more detailed data and confirm our findings.
Conclusions
In this study, the incidence rate of extensive subcutaneous emphysema was 2.05%, and approximately 75.58% of the cases occurred within 1 to 4 days postoperatively. In univariate analysis, patients with extensive subcutaneous emphysema were also likely to have a significantly lower body mass index, worse pulmonary function, greater intraoperative blood loss, longer time of operation, history of lung surgery, wider scope of surgery, and more extensive pleural adhesions. More importantly, segmentectomy, lobectomy, and extensive pleural adhesions were found to be independent risk factors for extensive subcutaneous emphysema after pulmonary resection through VATS.