Introduction
Atopic dermatitis (AD) is a chronic, recurrent inflammatory skin disorder affecting many people especially children. Clinically, it is marked by itching and dry skin, scratching, patchy eczema, exudation, and skin thickening and discoloration [1, 2]. The pathogenesis of AD is complicated and still not fully known. Typical histopathological features such as vesicle formation, perivascular inflammatory infiltration and epidermal spongiosis in the dermis are observed in AD [3]. In addition, Fas/Fas ligand-mediated keratinocyte apoptosis and caspase activation are included in the pathophysiology of AD [3].
Advanced glycation end products (AGEs) are a very different structure group of substances. The term “AGEs” includes all kinds of molecules e.g. proteins, lipid and nucleic acids [4]. The interaction between AGEs and their receptor (RAGE) reveals oxidative stress; thrombogenic and inflammatory reactions occur in various cells; they play a major role in the occurrence and advancement of destroying disorders, e.g. inflammatory, cardiovascular, neurodegenerative, autoimmune disorders as well as cancer growth and metastasis [5]. AGE belongs to the immunoglobulin superfamily which acts like a model-recognition receptor for various ligands including those glycated and non-glycated ones. Membrane-bound RAGE, the cleaved form soluble RAGE (sRAGE) behaves as a trap receptor by connecting pro-inflammatory ligands engaged towards mRAGE, providing anti-inflammatory effects (mRAGE) that induce activation of various downstream inflammatory pathways by binding to ligands [6]. In last decades, there has been growing evidence regarding the important role of oxidative stress in many skin aging and cutaneous diseases. The skin is highly sensitive to AGEs changes and that accumulation of AGEs in the skin leads to an increase in free radicals [7].
The apoptosis is programmed cell death which is an important process for tissue functions and it is also involved in wound healing by prevention of persistent inflammation. The Fas/Fas ligand interaction-mediated keratinocyte apoptosis and resultant caspase activation play a role in the pathogenesis of AD [3, 8]. The AGEs binding to RAGE leads to production of reactive oxygen species, which eventually results in apoptosis [9]. The AGEs can enhance fibroblast apoptosis via activation of various caspases including caspase-3 and caspase-8 [10]. An increase in AGE levels leads to prolonged inflammation, with an adverse effect on wound healing [11]. Besides, NF-κB is activated when AGEs bind to RAGE, resulting in cytokine activity. The enhanced cytokine activity prolongs inflammation with adverse effects on healing. It seems that it is needed to terminate the inflammatory phase in order to proceed to subsequent stages of wound healing [11].
In particular, RAGE levels are high in lung tissue, implying a major role for RAGE in pulmonary physiology. It is found in the alveolar and bronchial epithelia cells and primarily localized at basolateral membrane alveolar type 1 (AT1) cells. Currently, RAGE is thought to be an indicator for AT1 cells [12]. The accumulating evidence suggests that RAGE may have a key role in lung disorder including allergic airway inflammation, asthma, acute respiratory distress syndrome (ARDS), asthma or bronchopulmonary dysplasia [6, 13, 14]. Serum levels of sRAGE are reduced in patients with asthma and allergic airway disease, bronchiolitis, chronic obstructive pulmonary disease, adults with AD and psoriasis [13–18]. This is uncertain in paediatric patients with AD.
Aim
This study was intended to establish sRAGE levels in children with AD and to evaluate the relation between sRAGE and illness severity.
Material and methods
This randomized, prospective, case-control study was performed in the Department of Paediatric Allergy and Immunology outpatient clinic of a tertiary centre between March 2022 and October 2022. The major inclusion criterion was the presence of current AD in children aged 4–24 months (patient group). The AD was diagnosed based on the Hanifin and Rajka criteria [19]. Severity of AD was evaluated with the objective SCORAD index by the same allergist [20]. Patients who have additional chronic skin disease and any other disorder were excluded from the study. The children within the control group were brought to the Outpatient Clinic of the General Paediatrics Department for a routine healthy child follow-up in the same age as the patient group (control group). Patients who have any systemic disease or skin disorder were excluded from the study. In the study, a skin-prick test, white blood cell count, absolute neutrophil count, C-reactive protein levels, absolute eosinophil count, and a survey about the history of atopic disorders and the risk factors for AD were used. We excluded patients with chronic skin diseases other than AD and any other disorder. Demographic characteristics, clinical features, laboratory data were recorded in all children. Total IgE levels were evaluated by using sandwich immunoassay method on an autoanalyser (Roche Diagnostics E 602, Mannheim, Germany). and eosinophil count was analysed by using Sysmex-xn9000 haematology analyser. Venous blood samples were collected in test tubes without anticoagulant for determination of sRAGE. The blood samples were centrifuged at 4000 rpm for 10 min; afterwards, the sera were separated and stored at –80°C until analysis.
Ethical disclosures
The Clinical Research, Ethics Committee of the University of Health Sciences, Kayseri City Hospital approved the study (18.08.2022/691). Before participation, written informed consent was acquired from all parents or legal guardians.
Measuring gelsolin concentrations in plasma
Serum sRAGE levels were evaluated by human sRAGE enzyme linked immunosorbent assay (ELISA) kit (Range: 0.05–24 ng/ml, BTLAB, Shanghai, China). sRAGE levels were analysed in accordance to the manufacturer’s instructions and expressed as ng/ml. To calculate the sample concentrations, we used calibration curves obtained from the standard. Both intra- and inter-assay coefficients of variation were < 10%.
Statistical analysis
Descriptive statistics were made as number of units (n), percent (%), median (M), minimum (min.), maximum (max.) and interquartile range (IQR). The data of numerical variables were evaluated with the normal distribution Shapiro-Wilk test of normality. For numerical variables, two-group comparisons were made with the Mann-Whitney U test, and three-group comparisons were made with the Kruskal-Wallis analysis. If the result of the Kruskal-Wallis analysis was found to be significant, multiple comparisons were made with the Dunn-Bonferroni test. The distribution by gender was evaluated with the Pearson χ2 test. The performance of sRAGE in predicting inflammation was evaluated by Receiver Operating Characteristic (ROC) Curve analysis. Spearman correlation analysis was used to test the relationship between numerical variables. SPSS Statistics Standard Concurrent User V 26 (IBM Corp., Armonk, New York, ABD) was used for all the statistical analysis. A p-value < 0.05 was accepted as statistically important.
Results
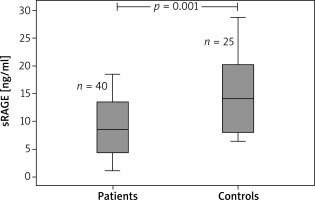
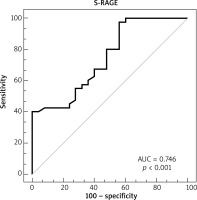
Overall, the study contained 65 children (40 children with AD and 25 age- and sex-matched healthy controls). The age range was 4–24 months. The median (IQR) age was 7 (8) and 10 (5) months in the patient and control groups, respectively. Table 1 displays the demographic features of the patients in the study. The eosinophil count and total IgE value were remarkably higher in the atopic group when compared to the non-atopic group and controls. White blood cell counts, absolute neutrophil counts, and c-reactive protein levels did not differ between groups (Table 2). The medians (IQR) of sRAGE levels in patient and control groups were 8.43 (17.33) and 14.09 (22.29), respectively (p < 0.001). The medians (IQR) of sRAGE levels in atopic, non-atopic and control groups were 8.5 (14.17), 7.75 (17.33) and 14.09 (22.29), respectively (p = 0.004) (Figure 1). We could not show any statistically remarkable distinction in patients with atopic and non-atopic groups (Table 2). No remarkable correlation was found between sRAGE levels, eosinophil count, and total IgE levels and O-SCORAD index. According to the receiver operating characteristic (ROC) curve analysis realized for sRAGE between the patient and control groups, the area below the curve was 0.746 and was statistically significant. When the sRAGE value was ≤ 17.27, the sensitivity value was 97.5% and the specificity value was 44.0% (Figure 2).
Table 1
Characteristics of the study population
| Parameter | Groups | P-value | |
|---|---|---|---|
| Patient group (n = 40) | Control group (n = 25) | ||
| Gender& n (%): | |||
| Girl | 16 (40.0) | 11 (44) | 0.800 |
| Boy | 24 (60.0) | 15 (56) | |
| Age† [month]: | |||
| M (interquartile range) | 7.0 (8.0) | 10 (5) | 0.131 |
| Min.–max. | 4–24 | 4–16 | |
| s-RAGE† [ng/ml]: | |||
| M (interquartile range) | 8.43 (9.22) | 14.09 | 0.001 |
| Min.–max. | 1.04–18.37 | (12.53) 6.35–8.64 | |
| IgE†: | |||
| M (interquartile range) | 20.97 (53.97) | 12.48 (33.27) | 0.107 |
| Eosinophil counts† [mm3]: | |||
| M (interquartile range) | 440 (315) | 210 (235) | < 0.001 |
Table 2
Comparisons between groups
Discussion
Our study indicated that sRAGE levels were lower in patients with AD than in the healthy controls. However, sRAGE levels did not differ significantly between patients with atopy positive and atopy negative. This result suggested no remarkable effect of atopy on sRAGE. No significant correlation was found between sRAGE levels and the severity of the disease. To the best of our knowledge, this is the first study showing lower sRAGE levels in patients with AD at childhood.
In clinical practice, aging phenotype is associated with more wrinkled and pigmented skin in patients with chronic AD when compared to controls. Moreover, it has also been recommended that AGEs act a role in the aging process given that the amount of AGEs is higher in elder individuals [21]. In animal models, it was shown that the recombinant soluble RAGE (sRAGE) blocked AGE-RAGE signalling pathway and that the exogenous sRAGE can remove AGEs from circulation and may prevent AGE-induced tissue damage via acting as a decoy receptor for AGEs [22]. Given the neutralizing role of sRAGE, it is proposed that the lower sRAGE level can increase AGE-RAGE reaction; thus, exacerbate subsequent inflammation. Exogenous sRAGE results in enhanced wound healing due to limitation of the inflammatory phase in the wound repair process [23]. In our study, sRAGE level was found to be lower in patients with AD. The treatment with sRAGE or ingredients containing sRAGE can reduce the symptoms of individuals with AD.
The production and accumulation of AGEs is a naturally occurring process during aging in the human tissues. This process is raised by persistent hyperlipidaemia, hyperglycaemia and oxidative stress often observed in patients with psoriasis. A study by Papagrigoraki et al. found raised levels of skin and circulating AGEs in psoriasis patients. This study involved 80 patients with mild/severe psoriasis and 80 controls (40 with severe eczema, 40 healthy individuals). Levels of cutaneous and serum AGEs were higher in patients with severe psoriasis compared to patients with mild psoriasis, patients with severe eczema and to healthy controls. In the same study skin AGEs levels correlated positively with serum AGEs in all patients and serum AGEs levels well correlated with disease severity in psoriatic patients [23].
By contrast, serum levels of RAGE were remarkably lower in patients with psoriasis compared to those with eczema or healthy individuals and RAGE levels inversely related with psoriasis area and disease severity index score [24]. Authors showed that cutaneous AGEs might be the link between dermal inflammation and an increased metabolic cardiovascular risk commonly seen in psoriasis patients. Also, it was shown that dysregulation in keratinocyte apoptosis is important in the pathogenesis of psoriasis [24]. These results recommend that the level of sRAGE, which has anti-inflammatory and anti-apoptotic effects, can be low in psoriatic cases which has intensive AGE accumulation. In accordance with the literature, we found low sRAGE levels in the patient group with AD, which is a kind of disease with inflammation and apoptosis in its etiopathogenesis.
Some articles have shown that a decreased RAGE level is related to neutrophilic airway inflammation in asthma and chronic obstructive pulmonary disease (COPD), it has been proposed that sRAGE might play a preventive role in inflammatory lung disorders [6, 16]. Chen et al. asserted that sRAGE heals cigarette smoke-induced airway inflammation via downregulating S100A8/A9 expression and its related immune-inflammatory reactions in the knockout of RAGE mice. This study hypothesized that reduced sRAGE may increase AGE-RAGE reaction and result in inflammation [25]. In the study by Zhang et al., sRAGE production was considerably reduced in the mouse model of neutrophilic asthma, which was strongly related with increased IL-17 levels and neutrophil infiltration, indicating a role for sRAGE production in the development of Th17 sided inflammation [15].
The sRAGE inhibits interactions between RAGE and AGE products like High Mobility Group Box 1 (HMGB1) by acting as a decoy receptor. Zang et al. showed that sRAGE inhibited Th17 polarization driven by recombinant HMGB1 (rHMGB1)-activated dendritic cells (DCs) in vitro [15]. Another study indicated that sRAGE levels was significantly lower in asthmatic patients when compared to controls; in addition, in subgroup analysis the sRAGE levels were also considerably lower in patients with severe asthma and uncontrolled asthma [26]. In a prospective, observational and analytical study by Egron et al., 93 infants were recruited. Authors reported that sRAGE levels were significantly lower in acute bronchiolitis patients than in controls but did not correlate with clinical severity. Also, no correlation was found between serum sRAGE and severity score, respiratory viruses, and recurrent wheezing at 1 year [27]. In our study, we found that the sRAGE levels were lower in patients with AD when compared to healthy controls in agreement with the literature; however, no association was detected with disease severity.
Hong et al. showed that AGE expression was higher in corneocytes in the cutaneous tissues of adult patients with AD; therefore, suggesting that AGE can be associated with more intense oxidative stress and facilitate dermal inflammation. They also found that the mean levels of corneocyte AGEs from the lesional and non-lesional areas did not differ significantly. This condition may underline the presence of subclinical inflammation in the non-lesional AD skin. Authors additionally demonstrated that serum sRAGE was prominently reduced in patients with AD [19]. sRAGE eliminates the harmful effects of AGE. For this reason, low levels of sRAGE have been recommended as a biomarker candidate to define the presence of some diseases such as asthma, allergic airway diseases, psoriasis, cardio-metabolic diseases, dyslipidaemia and cancer etc.
Our study has some limitations. First, it is a single-centre study and includes a small number of children and only reflects the data of our patients. Second, sRAGE levels in the skin tissue were not assessed in the study. Because of these limitations, our results may not be practicable to other institutions.
Conclusions
Based on these findings, it is asserted that sRAGE may play a role in the disease process in AD. The decrease in sRAGE may demonstrate ongoing inflammation and apoptosis. The sRAGE can be used as a marker for AD. Also, the topical and/or systemic use of some molecules (such as sRAGE and/or products containing sRAGE) may be a target of therapeutic interventions in the future.