Introduction
Chronic spontaneous urticaria (CSU) is a condition characterized by spontaneous appearance of pruritic hives, angioedema, or both that recur without an identifiable external cause and persist for at least 6 weeks [1]. It is estimated to affect 1% of the population worldwide. Adults are more likely to develop CSU than children with a peak occurrence in the third to fifth decades of life [2–4]. Also, prevalence in women is reported to be slightly higher than in men, especially in Europe and Northern America [2].
CSU seems to carry a substantial burden for affected patients as well as the health care system [2]. According to the international observational study among 673 adult patients with CSU whose symptoms persisted for ≥ 12 months despite treatment, this disease significantly impairs the quality of life including impact on sleep, daily activities, work performance and social interactions [5]. Increased disease severity corresponds with a higher incidence of those effects. The weekly Urticaria Severity Score (UAS7) and Dermatology Life Quality Index (DLQI) are two commonly used instruments to assess disease severity and its impact on the quality of life (QoL). UAS is a validated measure to assess disease activity in patients with CSU, which is recommended by the US and European guidelines to evaluate severity of the disease and response to treatment in day-to-day clinical practice. The UAS score (range: 0–6) comprises a sum of daily ratings for itch severity and number of hives (0–3 points for each). The weekly Urticaria Activity Score (UAS7) sums UAS scores during a 7-day period, and possible values for the UAS7 range from 0 to 42 [4, 6]. DLQI is a standardized scale that consists of 10 questions about patients’ everyday life in the previous week. The number of points represents the impact of skin disease on patients’ quality of life. This measurement can be used widely in different skin disorders including CSU [7].
According to current guidelines for diagnosis, management and therapy of urticaria, recommended treatment options aim to target mast cell mediators such as histamine, or activators, such as autoantibodies. Patients’ refractory to the first-line treatment – second-generation H1 antihistamines – require using targeted monoclonal antibodies for disease control. At present, omalizumab is the only FDA- approved biologic for CSU, which can be used in patients over 12 years of age [4]. It is a recombinant, humanised anti-immunoglobulin-E antibody used to control symptoms in active CSU [8, 9]. Omalizumab binds to the constant region of the IgE molecule, avoiding the interaction between free IgE and high- and low-affinity IgE receptors, which leads to a downregulation of high-affinity IgE receptor expression on inflammatory cells [3, 10]. In Poland omalizumab has been available for patients with CSU in a drug program B.107 funded by the Polish National Health Service since January 2020. One cycle includes 6 doses of omalizumab (300 mg/dose) administered subcutaneously once per 4 weeks for 24 weeks. Later patients are monitored for the relapse and in case of exacerbation of CSU (UAS7 ≥ 16) they can be re-enrolled to the drug program for the next cycle of treatment [11].
The efficacy of omalizumab has been well established in many randomised controlled trials of CSU including the 3 pivotal trials that led to its FDA approval involving 733 patients treated with various doses of omalizumab [1, 9–11]. However, there is still a need for real-world data confirming the quality of presented evidence. Due to that reason our single-centre retrospective cohort study was aimed to provide some real-world evidence considering the long-term efficacy and safety of omalizumab for treatment of refractory CSU in Poland.
Aim
The aim of our study was to perform a retrospective analysis of the patients with chronic spontaneous urticaria treated with omalizumab at the dermatology department to assess effectiveness of omalizumab therapy.
Material and methods
Patient recruitment
The medical records of the patients with CSU enrolled in the National Health Service’s drug program B.107 between January 2020 and June 2023 at the dermatology department were researched. Patients were enclosed to the study based on criteria from National Health Service’s drug program B.107. Inclusion criteria for the evaluated patients involved years of age ≥ 12, at least 6-month history of CSU prior to the admission, severe CSU (UAS7 ≥ 28 and DLQI ≥ 10) as well as lack of clinical improvement after treatment with second-generation H1 antihistamines taken at a dose 4 times higher than a standard one for a minimum of 4 weeks. Exclusion criteria included years of age ≤ 12, pregnancy or lactation and occurrence of any contraindications to the use of omalizumab. Ultimately, our study group consisted of 61 patients accepted to the drug program B.107.
Study design
According to the protocol, our patients were given 300 mg of omalizumab subcutaneously every 4 weeks for 24 weeks (in total 6 doses) and were later monitored for any clinical exacerbation. All laboratory and clinical evaluation results used in the study were routinely obtained during patient examinations and retrospectively retrieved from medical records. The patient’s data were analysed for age, gender, and duration of the disease before omalizumab administration. Disease severity as well as omalizumab effectiveness was assessed with Urticaria Activity Score (UAS7) at week 0, 4, 8, 12, 24 and Dermatology Life Quality Index (DLQI) at week 0, 12 and 24. Patients whose disease was exacerbated (UAS7 ≥ 16) during the follow-up after omalizumab withdrawal were readmitted to the program. In our study the number of readmissions to the drug program, interval between treatment cycles and effectiveness of omalizumab in each treatment course were also evaluated. All gathered data were statistically analysed to assess effectiveness of omalizumab therapy in each treatment cycle and to find a correlation which might affect efficacy of the drug administered.
Interpretation of scales results was presented in Table 1 [7, 12, 13].
Table 1
Two scales used in our research
Results
Sixty-one patients took part in our research. Among them there were 42 (68.9%) women and 19 (31.1%) men. Also, 4 (6.5%) children were included, the youngest was 13 years old and the oldest 16 years old. The mean age was 44.4 (range: 13–81 years). The mean duration of chronic spontaneous urticaria before omalizumab administration was 56.8 months (4.7 years); range: 6–371 months. One of the patients resigned from the drug program after 1 month of treatment because of exacerbation after omalizumab implementation.
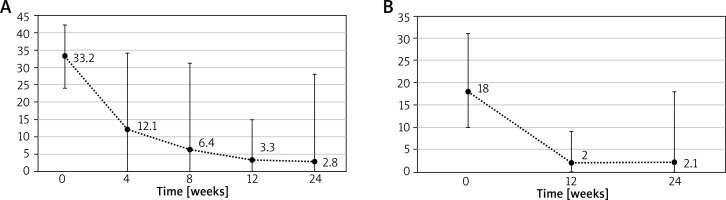
Sixty-one patients were included in the first cycle of treatment and 46 completed the whole cycle till the data analysis. During the cycle the mean UAS7 declined from 33.2 to 2.8 and mean DLQI from 18 to 2.1. Changes in disease severity scores over the first cycle are presented in the Figures 1 A, B.
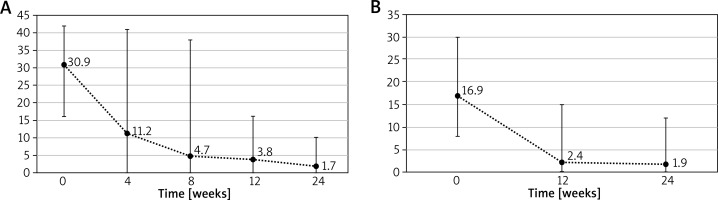
The mean period without exacerbation following omalizumab withdrawal after the first cycle was 74.1 weeks (range: 20–384 weeks). Due to deterioration in disease control 30 patients were re-enrolled for the second cycle of treatment and 24 completed it till the data analysis. At the beginning of the second cycle mean UAS7 was 30.9 and DLQI 16.9, which later decreased to 1.7 for UAS7 and 1.9 for DLQI in the 24th week of treatment. Changes in UAS7 and DLQI during the second cycle can be observed in Figures 2 A, B.
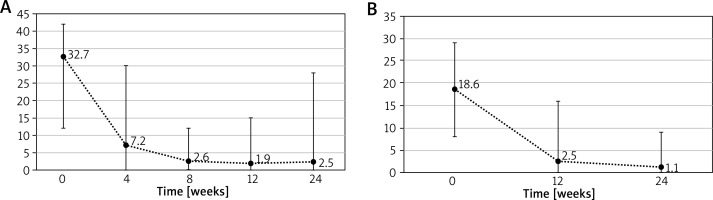
After the end of the second cycle of treatment the mean period without exacerbation was 72.9 weeks (range: 0–167 weeks). 16 patients were administered the third cycle of omalizumab therapy with initial mean UAS7 32.7 and mean DLQI 18.6. At the end of the third cycle mean UAS7 was reduced to 2.5 and mean DLQI to 1.1. The development of UAS7 and DLQI during the third cycle is shown in Figures 3 A, B.
Discussion
The current study demonstrated the long-term effectiveness of omalizumab in a real-world setting. Almost 64% of our patients achieved UAS7 ≤ 6 at week 12 of omalizumab treatment, indicative of well-controlled disease, which is in line with the results observed in the three pivotal studies (52–66% of patients with a UAS7 ≤ 6) [1, 10, 11]. Furthermore, the same trend was observed in our second (63.3% of patients with UAS7 ≤ 6 at week 12) and third (93.75% of patients with UAS7 ≤ 6 at week 12) course of treatment. Additionally, in each cycle we observed a similar significant decrease in UAS7 scores which indicates that CSU patients do not become resistant to omalizumab treatment. However, there was a group of patients whose state quickly exacerbated after omalizumab withdrawal and required readmission to the drug program. The mean time to the first relapse after omalizumab withdrawal was 74.1 weeks and to the second relapse 72.9 weeks, which are quite similar. These findings support the hypothesis that in some cases longer treatment may be needed to sustain the benefit of omalizumab on symptoms and QoL.
Our study shows that CSU has a moderate to extremely large impact on patients’ quality of life, which increased with the severity of the disease. The mean DLQI at the beginning of treatment was slightly higher than in pivotal studies that might relate to high initial severity scores [1, 10, 11]. Yet, in each cycle we observed a significant improvement in DLQI scores, which is in line with other research [1, 10, 11, 14, 15]. Furthermore, some patients experienced an improvement in the quality of life despite not responding fully to treatment. These findings suggest a subjectively high burden of the disease on everyday life. Even though some symptoms may still remain, the patient’s well-being and hope for improvement can influence DLQI scores.
Recently, Tarkowski et al. also provided an insight on omalizumab effectiveness in the National Health Service drug program among the Polish population. They reported that 53.57% of patients classified as responders displayed a noticeable response to the implemented treatment (UAS7 = 0 at week 24) and 33.93% of patients classified as non-responders had a weaker or no response (UAS7 > 0 at week 24). These numbers are in line with our results – 50.8% of patients were classified at week 24 of the first course of treatment as responders and 24.6% as non-responders. Furthermore, the mean UAS7 at week 0 was higher than ours – 41.48 vs. 33.2, but the mean UAS7 at the end of week 24 was analogous to ours – 2.56 vs. 2.8. In terms of DLQI, the outcomes were also quite similar – DLQI decreased from 19.21 to 1 vs. from 18 to 2.1. They also observed that the efficacy in the second cycle of treatment with omalizumab was even higher than in the first one, the same as in our study. Unfortunately, these data do not include information about the third course of treatment, which was provided in our research [15].
This research provides an important perspective on the topic, but there are several limitations that need to be acknowledged. Firstly, the study group was relatively small and included only patients who had moderate to severe urticaria at week 0. Secondly, a study design with several runs of omalizumab treatments led to that analysed patients were at the different stages of treatment which caused missing data from some patients. Thirdly, the data were gathered only in one centre in Poland, so further cross-sectional studies concerning this topic in different cities are needed to expand knowledge about effectiveness of omalizumab therapy in CSU patients in Poland.
Conclusions
Our findings confirm that omalizumab administered at the dosage of 300 mg in 4-week intervals significantly reduces symptoms in patients with chronic idiopathic urticaria who remained symptomatic despite the use of approved doses of H1-antihistamines. It also shows that omalizumab significantly improved severity of the disease and patients’ quality of life in each cycle of treatment, which indicates that CSU patients do not become resistant to administered therapy. In conclusion, this research supports long-term safety and effectiveness of omalizumab in CSU patients who responded inadequately to conventional therapies in real-world clinical practice in Poland.