Introduction
Skin diseases, also known as dermatoses, are a group of conditions with different causes, sometimes not fully understood, whose symptoms can manifest in all layers of the skin. The lesions that appear on the skin mostly proceed with local inflammation. Therefore, external formulations applied topically are often the first line of treatment for this type of condition. In recent years, azelaic acid (AZA), a natural acid applied topically, has become increasingly popular.
AZA is a substance naturally produced by the yeast Malassezia spp. [1]. In humans, it is synthesized by omega-oxidation from fatty acids and occurs as a physiological component in human urine [2]. It is found in small amounts in healthy individuals and in excess in the urine of patients with ketosis and those with congenital or acquired inability to β-oxidize monocarboxylic acids – dicarboxylic acidosis [3]. AZA is metabolized by β-oxidation ending in the formation of malonyl-CoA or acetyl-CoA. Structurally, it is a 1,7-heptanedicarboxylic acid having 9 carbon atoms (Figure 1). Due to its chemical structure, it is capable of inhibiting tyrosinase and, to a much greater extent, thioredoxin [4]. In addition, AZA is an inhibitor of many other oxidoreductive enzymes, including enzymes involved in DNA synthesis such as DNA polymerase and mitochondrial respiratory chain oxidoreductases [5, 6]. It is a potent inhibitor of microsomal 5a-reductase and also inhibits anaerobic glycolysis [7, 8]. In vitro, it is a scavenger of toxic oxygen species, especially the toxic free hydroxyl radical, and inhibits the activity of oxyradicals in cell cultures [9, 10]. It also inhibits the production of reactive oxygen species by neutrophils [11]. Some of these actions are involved in its anticancer properties [3].
AZA has a wide spectrum of effects including antibacterial, anti-inflammatory and reducing excessive keratinization. This compound is most often used in the treatment of acne vulgaris, rosacea and a group of conditions associated with hyperpigmentation. It is a safe preparation and does not have an aggressive effect on the skin, so it can also be used for reactive and sensitive skins [12].
Topical treatment – pharmacokinetics and mechanism of action
Topically applied AZA penetrates all layers of the skin [13].
Absorption after topical administration of AZA varies, depending on the formulation used, between 3% (ointment, emulsion) and 8% (gel) of the dose administered. AZA is excreted mainly in unchanged form in the urine. To a lesser extent, it undergoes mitochondrial metabolism to malonyl coenzyme A and acetyl coenzyme A [6].
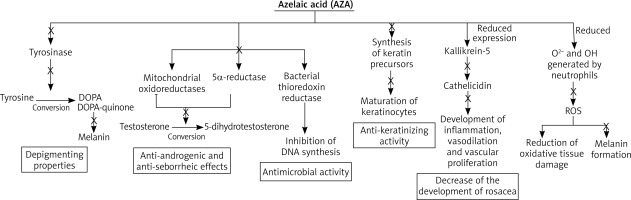
The molecular mechanism of action of AZA is broad, one of which is thioredoxin inhibition. The thioredoxin enzyme regulates tyrosinase activity through a feedback mechanism involving electron transfer to intracellular thioredoxin, followed by a specific interaction between reduced thioredoxin and tyrosinase [14, 15]. In addition, the thioredoxin/thioredoxin reductase system has been shown to be a major electron donor for DNA-regulating ribonucleotide reductases [16]. The inhibition of thioredoxin reductase by AZA provides a rationale for both its depigmenting properties and the reversible inhibition of DNA synthesis observed in cultured epidermal cells, as well as in some bacteria associated with acne vulgaris.
In addition, AZA directly affects tyrosinase through its competent inhibition [17]. This enzyme is involved in the conversion of tyrosine to DOPA and DOPA-quinone, which are precursors of melanin. Dicarboxylic acids do not affect normal skin melanocytes, so AZA can be used to treat many skin hyperpigmentations and does not cause discoloration of healthy skin near the lesions.
Another of AZA’s properties of action is its anti-infective and anti-inflammatory effects. AZA is a competitive inhibitor of mitochondrial oxidoreductases and 5α-reductase, inhibiting the conversion of testosterone to 5-dihydrotestosterone [18]. This results in anti-androgenic and anti-seborrheic effects. In addition, it also has bacteriostatic effects on aerobic as well as anaerobic bacteria [19]. The antimicrobial activity of AZA has been described by numerous authors, including against Propionibacterium acnes and Staphylococcus epidermidis [20, 21]. The mechanism of antimicrobial action is based on the inhibition of the enzyme thioredoxin reductase of bacteria which affects the inhibition of bacterial DNA synthesis that occurs in the cytoplasm [13].
AZA also exhibits anti-keratinizing activity through its antiproliferative cytostatic effect on keratinocytes affecting the modulation of the early and final phase of epidermal differentiation [22–24]. The antiproliferative effect is related to decreased DNA synthesis, mitochondrial damage, and expansion of the rough endoplasmic reticulum. In addition, AZA inhibits the maturation of keratinocytes by reducing the synthesis of keratin precursors [25]. The regulation of keratinization processes in the epidermis produces an anti-epidermal effect, which is currently used in dermatology.
In the presence of AZA, reduced expression of kallikrein-5 (a serine protease) and cathelicidin (an antimicrobial peptide) is observed [26]. This reduces the development of rosacea – kallikrein-5 causes an increase in cathelicidin, followed by the development of inflammation, vasodilation and vascular proliferation. AZA competently inhibits tyrosinase, which causes discoloration in the course of dandruff. This enzyme is involved in the conversion of tyrosine to DOPA and DOPA-quinone, which are precursors of melanin. Dicarboxylic acids do not affect normal skin melanocytes, so AZA can be used to treat many skin hyperpigmentations and does not cause discoloration of healthy skin near the lesions.
AZA also significantly reduces O2- and OH generated by neutrophils. It has an inhibitory effect on reactive oxygen species (ROS) generated by neutrophils, leading to a reduction in both oxidative tissue damage at sites of inflammation and melanin formation. This mechanism is relevant to hyperpigmentation and acne, which are more or less pathogenetically related to reactive oxygen species (ROS) [27]. These mechanisms are shown in Figure 2.
Dermatological applications of AZA in the treatment of dermatoses
AZA has found use in the treatment of skin diseases such as rosacea, acne vulgaris, hyperpigmentation, and the use of AZA has been documented in the treatment of hidradenitis suppurativa (HS), male pattern baldness (androgenic alopecia), perioral inflammation, psoriasis vulgaris and keratosis pilaris [28–32].
Rosacea
Rosacea is a chronic inflammatory dermatosis that mainly involves the central region of the face; it occurs on the nose, cheeks, forehead and chin [33–36]. The condition is characterized by recurrent redness, persistent erythema and the presence of papulopustular lesions and telangiectasias. The pathophysiology of this condition is not precisely known. Likely, genetic factors, immune dysregulation, vascular dysfunction and microorganisms such as Demodex folliculorum play a role in the development of the disease. Symptoms can be exacerbated after exposure to heat, stress, ultraviolet radiation, and after consuming spicy foods, alcohol or hot drinks [37–40]. AZA is a well-recognized agent used for the treatment of rosacea due to its anti-inflammatory and antioxidant properties, by inhibiting the production of reactive oxygen species and lowering cathelicidin levels through kallikrein-5 inhibition [39].
The effects of AZA have been confirmed in a number of clinical trials. In the largest study conducted by Draelos et al., 961 patients with the papulopustular variety of rosacea used a foam with 15% AZA (a form not available in Poland) twice a day for 12 weeks [41]. Compared to placebo, the foam with 15% AZA was more effective (32.0% vs. 23.5%; p < 0.001). A study by Thiboutot et al. found that topically applied AZA 15% gel in patients with papulopustular rosacea was more effective in reducing erythema and inflammation than placebo gel treatment [42]. In two separate studies, topically applied 15% AZA was shown to be more effective than 0.75% and 1% and metronidazole gel, respectively, in reducing the percentage of inflammatory lesions and erythema [43, 44]. The efficacy of the metronidazole gel reached a plateau, while the AZA gel still showed improvement after 15 weeks of follow-up. The main side effects of AZA in the reported studies were minor, transient pinching, burning or itching sensations.
Acne vulgaris
Acne vulgaris is one of the most common sebaceous gland disorders occurring mainly on the skin of the face, chest and back [45]. It affects 80–100% of people between the ages of 11 and 30 [46]. Hyperkeratinization, colonization of sebaceous glands by Cutibacterium acnes, excessive sebum secretion by androgen-stimulated sebaceous glands and inflammation play a major role in the formation of acne lesions [47]. In a study published by Thielitz et al. involving 55 patients with acne vulgaris, 15% AZA applied twice daily for 9 months was significantly more effective in treating inflammatory lesions than 15% AZA applied twice daily for 3 months and 0.1% adapalene gel applied once daily for another 6 months [48]. In a study by Katsambas et al., a cream with 20% AZA was as effective in treating comedonal acne as a cream with 0.05% tretinoin, while AZA had fewer topical side effects than a topical retinoid. In another study published by Picosse et al., AZA was more effective than placebo, especially on inflammatory lesions [49]. According to the guidelines of the Polish Society of Dermatology, AZA can be used twice daily. It is used in combination therapy, mainly with oral antibiotics, and because it does not induce antibiotic resistance it can be used in monotherapy [46]. The 2016 guidelines of the European Academy of Dermatology and Venereology recommend the use of AZA for the treatment of papulopustular acne of mild to moderate severity in monotherapy or in combination therapy with other treatments. AZA is also suitable for maintenance therapy [50].
Melasma
Melasma is a common, acquired and limited hyperpigmentation of normally sun-exposed skin and most often affects people with darker skin phototypes (Fitzpatrick IV to VI). It is characterized by symmetrical hyperpigmented patches occurring mainly on the cheeks, forehead and chin, but lesions can also occur in other sun-exposed areas. Melasma most often affects people of Asian and Hispanic origin. In addition to the main factor of exposure to ultraviolet radiation, other factors affecting the development of melasma include genetic predisposition, photosensitizing and anticonvulsant drugs, certain cosmetics and thyroid or ovarian disorders [51].
The largest study conducted by Balińa and Graupe compared the efficacy of a 20% AZA cream and a 2% hydroquinone cream applied twice daily for 24 weeks. The study proved the high efficacy of both formulations in terms of reducing pigment intensity and lesion size [52].
In contrast, in an older study published by Verallo-Rowell et al. among 155 patients using the formulations twice a day for 24 weeks, some were treated with 20% AZA (n = 77), while the rest were treated with 2% hydroquinone cream (n = 78). Seventy-three percent of patients using AZA cream achieved beneficial effects, while the percentage was lower at 19% in the hydroquinone-treated group. Reported side effects occurred at the beginning of therapy and were mild to moderate for both drugs [53].
In a similar study by Tehrani et al., 64 patients were divided into two groups [54]. Patients in the first group used a formulation containing 20% AZA and 5% hydroquinone, while the second group of patients used a formulation containing only 5% hydroquinone. Both formulations were applied once a day for 16 weeks. More favourable effects and a faster onset of action were observed with the combined formulation, while a higher rate of side effects (50%) was observed compared to the group of patients using the hydroquinone-only formulation (35%). This study proves that both preparations are effective, however, they should not be used in combination therapy without extreme caution.
This is supported by a study by Dayal et al. where a greater reduction in Melasma Area Severity Index (MASI) was observed when using a combination of 20% AZA and glycolic acid, rather than AZA alone [55].
Other uses of AZA in dermatology
The efficacy of AZA has also been confirmed in clinical studies on male pattern baldness and follicular keratosis. In a study conducted by Gugle et al., 46 patients with male pattern baldness were divided into two groups [32]. Twenty-three patients were treated with a topical 5% minoxidil lotion, while the others were treated with a lotion containing 5% minoxidil, 1.5% AZA and 0.01% tretinoin. There was statistically significant hair growth and thickening in both groups, while no significant statistical differences were observed between the groups. In a clinical trial involving 45 patients with follicular keratosis, they used 20% AZA cream on one arm, cheek or leg for 3 months and Cetaphil cream on the same areas on the opposite side of the body [28]. 92% of the skin treated with AZA and 83% of the skin to which Cetaphil was applied improved significantly in hyperkeratosis and/or erythema.
Prospects for the use of AZA in skin cancer
At present, there are few studies on the use of AZA in skin cancers, so extreme caution should be exercised in this group of patients. Currently, AZA is not routinely used in anti-cancer therapies, but studies conducted to date have revealed that it may be a future direction.
Promising results have been observed in lentigo melanoma as AZA selectively inhibited hyperactive or malignant melanocytes [56]. In a study of 23 patients with malignant melanoma, including some with metastasis and patients in terminal condition, topical and oral (10–15 g daily) administration of AZA for 1–12 weeks prior to surgical excision of the lesions resulted in arrest and subsequent regression of the progressive edges of the lesions [56]. A reduction in the size and flattening of the nodular areas, as well as a gradual lightening of pigmentation, was also observed. A study by Nazzaro-Porro et al. showed that topical AZA led to complete clinical and histological resolution of lentigo maligna in more than 50 patients [57]. The therapeutic results are durable, with 27 of the 50 patients being disease-free for 5–10 years after treatment. There was a relapse in 11 cases, but in all cases it resolved after resumption of treatment. In a similar clinical trial conducted by Leibl et al., AZA was used in 7 patients with melanoma, with remission observed in all [58]. In contrast, in a clinical trial conducted by McLean et al., of 9 patients treated with topical AZA, clinical improvement was observed in 4 patients and complete resolution of symptoms in one. However, 2 patients developed invasive melanoma during treatment [59].
Another interesting clinical case was a patient with malignant melanoma in situ and squamous cell carcinoma arising in a lupus vulgaris scar, where topical AZA therapy resulted in significant histologic improvement of the melanoma [60].
However, the therapeutic use of AZA in skin cancers has been limited due to poor absorption through the skin, thus requiring repeated applications to maintain long-term activity levels. This was mainly due to the low water solubility of AZA, which was one of the main difficulties. In addition, side effects such as local skin irritation, tingling and redness limited its use. However, there has been no study with formulations using liposomal technology. The potential role as a therapy for primary melanoma, and possibly other cancers, remains to be explored.
Currently, among the preparations available on the market, we can distinguish not only creams, but also newer forms of the drug in the form of liposomal gels (Table 1).
Table 1
Examples of azelaic acid preparations available on the market
Liposomal technology
Structure of liposomes
Liposomes are small, artificially produced vesicles that are characterized by their spherical shape. They show structural similarity to the cell membrane [61]. They are composed of one or more phospholipid bilayers, which are separated by aqueous spaces. They have a hydrophobic tail and a hydrophilic head, making their structure amphiphilic [62].
The tail chains are attached to the polar part by glycerol. The polar part is mainly an orthophosphoric acid residue bound to another water-soluble molecule. In an aqueous environment, a bilayer structure is spontaneously formed from phospholipids, with hydrophilic heads forming the outer layer [63]. The resultant vesicles, characterized by the presence of one or more phospholipid bilayers, exhibit the capacity to facilitate the transport of both hydrophobic substances, residing within the confines of the phospholipid bilayer, and hydrophilic substances, which traverse the aqueous space enclosed by said bilayers. To deliver the transported substances to the site of action, the phospholipid bilayer of the liposome interfaces with other bilayers such as the skin [64, 65].
Advantages and limitations of liposomal technology
The use of liposomes to support drug delivery has already had a major impact in many biomedical fields. The unique ability of liposomal systems to retain both lipophilic and hydrophilic compounds allows a wide range of drugs to be encapsulated by these vesicles [66]. They have been shown to be beneficial for stabilizing therapeutic compounds, overcoming obstacles to cellular and tissue absorption, and improving the biodistribution of compounds to target sites [66–68]. As a drug delivery system, liposomes offer several advantages, including biocompatibility, the ability to carry large drug payloads and a wide range of physicochemical and biophysical properties that can be modified to control their biological properties [68]. This enables the efficient delivery of compounds to target sites while minimizing systemic toxicity. Encapsulation of the drug in liposomes prevents early inactivation, degradation and concentration changes in the circulation [69].
Despite the many advantages of liposomes in in vivo studies, implementation of liposomes into clinical treatment has been slow. A limitation of this technology is the fact that liposomes are not as immunologically inert as once suggested [70]. Some liposomal systems are able to induce an innate immune response, with subsequent activation of the complement system. The result is the induction of an acute hypersensitivity syndrome known as complement activation-related pseudoallergy (CARPA) [71]. CARPA has been reported with experimental as well as clinically approved liposomal formulations (e.g., Doxil, AmBisome and DaunoXome) [71]. CARPA symptoms can manifest as anaphylaxis, facial redness and swelling, headache, chills and cardiopulmonary disturbances. Therefore, some liposomal systems should be used with extreme caution in cardiac patients [72]. General clinical management includes slowing down the infusion rate, as well as the use of standard anti-allergic drugs (such as antihistamines).
Application of liposomal technology
Due to their unique properties, such as non-toxicity and biodegradability, liposomes are increasingly being used in fields such as medicine, pharmacology, cosmetology, as well as the food and agricultural industries [73]. The encapsulation of an active ingredient in liposome form increases its penetration into the skin, solubility or stability. In addition, control over the pharmacokinetics and pharmacodynamics of the administered substance is increased [74]. Liposomes in cosmetics and dermatology act as a carrier mechanism for active ingredients. They deliver them directly to the cellular level, which allows their frequent use in these fields [64]. One of the most common ways of applying drugs to the skin is topical application. However, the use of conventional creams or ointments does not guarantee delivery of the active ingredient to the target structure in optimal concentration [75]. The key step in transdermal drug delivery is to overcome the skin barrier, the stratum corneum. To accomplish this, the molecule must have the appropriate physicochemical properties (lightness, solubility in the oil and aqueous phases, adequate distribution coefficient and appropriate melting point). Only a few substances meet these requirements, which will result in a significant reduction in the penetration of the active substance. Encapsulation of the active substance in a liposomal vesicle can help overcome these limitations. This is justified by the small size of liposomes and the similarity of the lipid composition of liposomes and keratinocytes, as well as the membranes of intercellular plaques. This enables liposomes to penetrate the epidermal barrier to a greater extent than other drug forms [76]. The effect of encapsulating the active ingredient in a liposomal vesicle may result in increased absorption of the drug into the epidermis, which will result in much longer sustained drug release and reduced drug absorption into the blood. In addition, this form allows the active ingredient to be protected from degradation, thus providing increased stability. This can lead to reduction of adverse effects, increased treatment efficacy and greater patient compliance. Currently, the use of liposomes in dermatology is most common in the treatment of conditions such as acne, atopic dermatitis, psoriasis and xerosis.
Liposomes are widely used in antifungal, antimicrobial and antiviral therapy. The best known and most widely used antifungal agent administered in liposome form is amphotericin B [77]. The drug itself is highly toxic, but administration in liposome form significantly reduces toxicity and dramatically reduces its accumulation in organs. More detailed studies are currently being conducted on the efficacy of antiviral drugs (such as ribavirin, acyclovir) encapsulated in liposomes, but it has already been shown that administration of these drugs in this form reduces their toxicity [65].
Antimicrobials encapsulated in the form of liposomes are less prone to enzymatic degradation [78]. This is particularly important for antibiotics such as cephalosporins and penicillins, which are sensitive to β-lactamases produced by some microorganisms. In addition, the amount of drug administered is reduced due to the increased cellular uptake of the antibiotic by microorganisms. This is due to the lipid nature of the vesicles. Liposomes are also used to treat HIV infection [79, 80]. Antiretroviral nucleotide analogues have been developed that are able to inhibit the enzyme reverse transcriptase, an enzyme essential for HIV multiplication. The agents in question exhibit dose-dependent toxicity. Encapsulating such drugs in the form of a liposomal vesicle reduces the amount of drug dose used, which reduces the occurrence of toxicity. Moreover, liposomal carriers are preferentially captured by virus cells, unlike human cells.
Liposomes represent the first generation of nanomedicine drugs accepted for cancer treatment. It is associated with increased penetration and prolonged retention of the compound at the target site. This phenomenon is called the EPR (enhanced permeability and retention) effect. Thanks to the EPR effect, when liposomes circulate in the bloodstream for a sufficiently long time, they are passively delivered to the tissues of patients. Thus, drug accumulation occurs to a greater extent in diseased tissues compared to healthy tissues. One of the better known drugs administered in the form of liposomes is Doxorubicin (Doxil), administered intravenously for Kaposi’s sarcoma, ovarian cancer, and breast cancer [81–84]. Currently, cationic liposomes are the most commonly used carrier for gene therapy. The factor that determines the effectiveness of using this type of liposomes is the interaction of the positively charged head group of phospholipids (components of the lipid bilayer) with the negatively charged DNA molecule. The encapsulation of the genetic material in the vesicles allows the DNA plasmid to condense into a highly organized structure, and thus protects the DNA from degradation during circulation in the body or during storage [85]. Another area of application is the use of liposomes as radiopharmaceuticals and radiodiagnostic carriers. Phospholipid vesicles are used as carriers for contrast agents. Their structure and properties allow the delivery of contrast to the tissues or organs under study. In addition, they increase the signal between the background and the area of interest.
Application of liposomal technology in preparations containing AZA
Liposomal technology has found its way into preparations containing AZA, where its efficacy and better bioavailability of the active ingredient have been proven [86]. Finacea/Skinoren Gel (15%), which was developed to meet the needs of acne patients with oily skin, was the first to gain popularity on the market [87]. Schering conducted two Phase II/III clinical trials with Skinoren Gel controlled by a product without AZA (“placebo”) and 5% benzoyl peroxide hydrogel, respectively. In addition, Schering conducted a phase II study between Skinoren Gel and Skinoren Cream in small groups of 15 patients each to highlight clinical efficacy [88].
Testing the penetration ability of active ingredients in test formulations is possible through the use of in vitro diffusion models [89]. One such study is the Franz chamber study. It has become a major research methodology that determines the relationship between drug, skin and formulation. Franz diffusion cell studies typically use human skin, animal skin or a synthetic membrane [90]. This research methodology allows comparison of different pharmaceutical forms of the drug as in the case of different forms of AZA preparations. Burchacka et al. conducted a study in which the drug-hydrogel liposomal and cream (active ingredient-AZA) forms were compared [86]. The study showed that in Franz’s cellular diffusion system, the liposomal gel formulation showed a higher concentration of AZA in the stratum corneum compared to the standard non-liposomal product [86]. Liposomal hydrogel formulations have been confirmed to provide better API bioavailability compared to other commercially available formulations. This is due to the fact that such liposomal formulations accumulate to a much greater extent in the stratum corneum of the epidermis. This makes it possible to significantly reduce the concentration of API, while achieving the same therapeutic effect that is demonstrated by preparations in other pharmaceutical forms.
On the Polish market at present, the only formulation using liposomal technology in the form of lipogel is Hascoderm lipogel. The 10% lipogel formulation shows very high accumulation (187.5 µg/cm2) of the active ingredient in the stratum corneum, which makes it possible to reduce the API content to 10%. Interestingly, the results of an in vitro antimicrobial preservation test showed that the lipogel formulation exhibits strong antimicrobial activity, so no preservatives are required for the final formulation [86].
Summary
Topical application of AZA has found wide application in the treatment of dermatoses. New formulation technologies using liposomes have made it possible to deliver the drug to all layers of the skin while accumulating AZA in the stratum corneum. This provides new opportunities in dermatological treatment and allows optimization of treatment.