Introduction
Severe acute respiratory coronavirus 2 (SARS-CoV-2), the pathogen responsible for the COVID-19 outbreak, is one of seven known human coronaviruses, which were first discovered in 1965 [1]. SARS-CoV-2 has four structural proteins: a spike (S), an envelope (E), a membrane (M), and a nucleocapsid (N). The S and N proteins are the key viral structural proteins and the main targets of antibody response after infection. The S protein is divided into two units – S1 and S2 – which are responsible for entry into the host cell [2]. The antibody response to these spike protein units was used to develop the neutralizing antibody (NAb) COVID-19 tests. Antibodies play an essential role in communicating the presence of a pathogen to immune effector cells. NAbs can activate immune cells, such as dendritic cells, T-cells, B-cells, and other mechanisms of the immune system. Huang et al. [3] reviewed 491 papers related to human coronavirus immunity and reported that MERS-CoV neutralizing antibodies were detected up to 60 weeks after symptom onset. Cao et al. [4] described the long-term dynamics for immunoglobulin G (IgG) and NAbs over the course of a 3-year study on SARS-CoV. The SARS-CoV-2 virus genome is 74.5% similar to that of the SARS-CoV genome [5] and 50% similar to that of the MERS-CoV genome [6]. According to Deng et al. [7], primary SARS-CoV-2 exposure protects against reinfection, which is important in the context of acquiring herd immunity. The COVID-19 immunoglobulin M (IgM)-anti-body response occurred earlier than the IgG response (4 days vs. 7 days); the IgM antibodies reached a peak on day 20, while the IgG antibodies peaked on day 25. However, IgM is detectable 3 weeks after infection, while the IgG response is maintained for much longer [8]. Long et al. [9] reported higher IgG and IgM titers in the group of patients with severe disease than in those with a mild course. In relation to the research conducted so far, it seems that the immunity against SARS-CoV-2 is sustained for a long period of time. Duysburgh et al. [10] reported the presence of NAbs in 91% of health workers at least 120 days after the onset of the first symptoms. The COVID-19 pandemic is the first one in which we have the opportunity for widespread use of a mRNA vaccination as a weapon against the virus [11]. These modern mRNA-based vaccines have proven to be a breakthrough in the fight against pathogens because the time from research to commercial use is measured in months, not years [12]. SARS-CoV-2 glycoprotein S receptor-binding domain (RBD) mutations are leading to an escape from the protective potential of vaccines, which is a major threat, especially in the most vulnerable populations [13]. Knowledge of the SARS-CoV-2 antibodies’ disappearance would help us identify the need for and the time of intervals between booster vaccinations.
The main aim of the study was to assess NAbs’ half-life time and to investigate the level of antibodies in a group of patients 12 months after the onset of COVID-19.
Material and methods
This single-center prospective observational cohort study of 38 patients took place in the Central Clinical Hospital of the Ministry of Internal Affairs in Warsaw, Poland.
Participants
The 38 patients participating in this observational study had COVID-19; 34 were men (87.2%) and 4 were women (12.8%). The patients were COVID-19 convalescents who were donating plasma. The eligibility criteria were in accordance with the Minister of Health’s regulations; a positive test for SARS-CoV-2 was required (real-time PCR method for SARS-CoV-2 RNA detection), as was a double-negative SARS-CoV-2 test with a minimum 24-h interval between the two tests. In this study, we assumed that patients with a mild form of the disease did not require hospitalization, as opposed to those with moderate or severe disease. Accordingly, 10 of them had developed a moderate or severe course of illness, while 28 had mild symptoms. Observation of NAbs took place from March 15, 2020 to June 26, 2021.
Methods
The first measurement of antibodies was made at least 2 weeks after recovery (confirmed by two negative PCR tests), but no later than 8 weeks after recovery. NAb level was measured using a DiaSorin Liaison SARS-CoV-2 S1/S2 IgG test for quantitative determination of IgG S1 and IgG S2 antibodies specific to SARS-CoV-2 – an indirect chemiluminescence immunoassay (negative values were < 15 AU/ml). Subsequent measurements took place at 2-month intervals. The observation period lasted 12 months; vaccination against COVID-19 finished the observation. Patients who received convalescent plasma treatment were not included in the study. The Bioethics Committee granted approval for this study (agreement number 40/2020 from April 3, 2020).
Statistical analysis
The levels of antibodies at different time-points are presented as continuous variables and as dichotomous variables (≤ 15 and > 15). Half-life was calculated using the pkexamine procedure in STATA 9.2 (Stata Corporation, College Station, TX, USA). The normality of the distribution was verified with the Shapiro-Wilk test, based on a visual assessment of histograms and the values of skewness and kurtosis. Because the data were not normally distributed, the levels of antibodies are presented as medians (Q1; Q3) with 95% confidence intervals (CIs). The frequencies of antibodies at a level greater than 15 are presented as counts (n [% frequency]) with 95% confidence intervals for proportions, using binomial exact calculation. All values below the lower detection limit were replaced by half of the corresponding detection limit. Also, all values above the upper detection limit were replaced by the product of the upper detection limit value and a factor of 1.2 [14]. Additionally, the percentage change in antibody levels vs. baseline levels was calculated, also with 95% CIs. Finally, the correlation between age and antibody level at different time-points as well as the change vs. baseline were verified using Spearman’s correlation coefficient. The statistical analysis was conducted with the software package R, version 4.0.5 (http://cran.r-project.org).
Results
The study group included 38 patients – 34 men (89.5%) and 4 women (10.5%). The median age in the group was 31 years (27-39) with a range of 22 to 67 years.
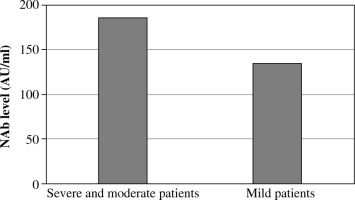
Ten patients (26.3%) had moderate or severe COVID-19 (they required hospitalization and oxygen therapy); 28 of the patients (73.7%) had mild symptoms of COVID-19. The mean level of neutralizing antibodies on first measurement was 134.5 AU/ml in the group of patients with mild symptoms and 186.4 AU/ml in the group of patients with a moderate or severe course of illness (Fig. 3).
Table 1
Antibody levels in the study group
Table 2
Antibody half-life
| N | Median (Q1; Q3) | 95% CI for median | Range | |
|---|---|---|---|---|
| Antibody half-life (months) | 38 | 5.8443 (4.2667; 9.1299) | 5.04305-7.298629 | 1.8439-27.1701 |
Fig. 2
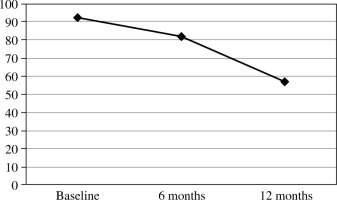
Percentage change in the number of patients testing positive for SARS-CoV-2 neutralizing antibodies
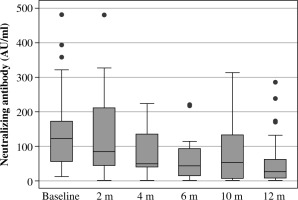
The median level of neutralizing antibodies in the first measurement was 122 AU/ml; for the last measurement it was 26.5 AU/ml.
As can be seen, the median antibody half-life in our group of patients was 5.8443 months (range: 1.8439 to 27.1701).
There was no significant correlation between age and antibody level, nor in the change in antibody level vs. baseline (p > 0.05 for all time-points; Table 3).
Discussion
In this study we evaluated the change of the SARS-CoV-2 neutralizing antibody level in a one-year period. Globally, societies are trying to overcome the SARS-CoV-2 pandemic with the use of recently developed vaccines, which present a strong immune response against the virus [15, 16]. Unfortunately, despite the availability of effective vaccinations, a part of the population will not use this method of prevention. According to Zhao et al. [17], the humoral response seems to have a significant influence on protection against COVID-19. In contrast to the specific IgM, which disappears around 12 weeks after the onset of symptoms [18], IgG has a much longer duration. Previous studies found high titers of NAbs even 6 months after the onset of COVID-19 symptoms [19].
Among the 38 patients, 21 (56.75%) presented a protective level of SARS-CoV-2 antibodies 12 months after infection. Liu et al. [20] found a difference between disease severity and antibody level, which was also visible in our study. Despite the difference between age and disease severity [21], we found no significant correlation between age and antibody level. However, our study revealed a correlation between disease severity and antibody level, which can be found in other studies [22]. According to our findings, the protective antibody level seems to be high for even longer than one year for most convalescent patients, but more research is needed to prove it. The fact that not all patients presented at the ideal time was a significant obstacle; therefore, the study assumed a one-month tolerance period.
The important variable to be determined is the protective level of NAbs. Khoury et al. [23] determined that the estimated 50% protective neutralization level (which equates to a 50% lower risk of getting sick) was 20.2% of the mean convalescent level. According to these findings, our convalescents’ 50% protective neutralization level was 34.37 AU/l. This level was maintained in 70% of our patients after 6 months of observation, and in 32% of them after 12 months.
Our study showed no significant correlation between age and SARS-CoV-2 neutralization antibody level, but most available studies show much higher mortality in the group of elderly patients [24, 25]. The greater number of comorbidities appears to be the most likely cause of the increased mortality in this group of patients. Moreover, nutritional status seems to play a crucial role in the defense against the virus; patients with nutritional deficiencies seems to be more susceptible to infectious diseases, which can lead to harmful consequences [26]. Our research results presented in this article prove that the mean level of neutralizing antibodies was higher in the group of patients with a moderate or severe course of COVID-19 illness than in the group of patients with mild symptoms (186.4 AU/ml vs. 134.5 AU/ml). The anti-gen specific T cell response also appears to play a large role in COVID-19 immunity. However, T-cell response could be different from the SARS-CoV-2 neutralizing antibody level. Studies published so far show that SARS-CoV-2-specific CD8+ and CD4+ T cells were identified in 70% and 100% of COVID-19 patients, respectively [27]. According to Ibarrondo et al. [28], the kinetics of neutralizing antibodies after vaccination is very similar to that observed after natural infection. However, NAb levels decline more rapidly in vaccinated people with no history of SARS-CoV-2 infection. Dimeglio et al. found a difference in NAb kinetics in two groups of patients: vaccinated people with no history of infection and vaccinated people with previous infection. In the first group, the protection time was approximately 309 days, while in the second group it was 714 days [29]. Our research team previously reported that among COVID-19 patients, comorbidities such as active cancer, hypertension, arrhythmia, and cardiac insufficiency correlated with a higher mortality rate [30]. The fact that our patients were infected with the primal strain of the SARS-CoV-2 virus seem to be a limitation in our study. However, recent studies relating to the newest strains such as Omicron prove the same protection period [31]. Further assessment of NAb levels in the Polish population has been hampered by the fact that 47% of the population had received at least the first dose of a COVID-19 vaccination by July 27, 2021. Our group of patients seems to extrapolate to the population of vaccinated patients. It is worth noting that the type of selected vaccine can make a great difference. According to Rogliani et al. [32], adenovirus-vector-based, mRNA-based, and inactivated SARS-CoV-2 vaccines are superior to the plant-derived virus-like particle and SARS-CoV-2 recombinant spike glycoprotein nanoparticle vaccines. Additionally, the CD8+ T cell response was eliciting relatively narrowly in the group of vaccinated patients compared to patients suffering from COVID-19 [27].
Conclusions
Our latest results along with the publications cited above prove that the SARS-CoV-2 vaccination booster should be indicated, especially for patients with risk factors such as advanced age and comorbidities. Due to the proven antibody half-life, it seems reasonable to use such vaccinations at least once a year, more often in high-risk groups. Moreover, patients with a history of COVID-19 disease will get the most benefits.