Summary
The relation between uric acid level and blood pressure is not clear. This study aimed to investigate the relationship between uric acid level and BP in patients hospitalized in an internal medicine ward. We analyzed data of 561 patients and did not confirm an independent relation between uric acid level and blood pressure, nor between allopurinol treatment and blood pressure.
Introduction
Uric acid (UA) is the end product in the metabolism of purine nucleosides, which constitute nucleic acids. Normal values for plasma UA range between 3 and 7 mg/dl (180–420 μmol/l). Regarding the abnormal values, an abnormally high UA level is of particular clinical importance and is diagnosed as hyperuricemia.
There are many causes of hyperuricemia – both an increased supply of purines and decreased elimination of their metabolites can result in high UA levels [1, 2]. Regarding the causes of hyperuricemia, renal or gastrointestinal impairment of UA elimination should be mentioned. In humans suffering from kidney diseases, processes such as glomerular filtration and urate excretion are limited [1, 3]. The causes of hyperuricemia frequently observed in internal departments are of metabolic backgrounds such as obesity, insulin resistance, and dyslipidemia [1, 2, 4]. Sex is yet another factor that should be considered when assessing the causes of hyperuricemia. UA levels may be higher in men and women after menopause. In women after menopause estrogen levels, which have an uricosuric effect, substantially decrease [1, 2]. Some medicines can also contribute to elevated UA levels [1, 2].
UA is not only a superfluous product of purine nucleotide metabolism but also exhibits biological activity. Its effects on the human body can be observed as both beneficial and potentially pathogenic. Hyperuricemia is a prerequisite for the development of gout [2]. UA is also a powerful antioxidant, which may play a role in the pathogenesis of cardiovascular diseases. Due to its antioxidant properties, its compensatory role in reducing oxidative stress and protective effects on the vasculature has been postulated [1, 5]. On the other hand, the results of several studies and meta-analyses suggested that high UA level is a risk factor for cardiovascular (CV) events [1–4, 6–9]. One could also argue that high UA is a marker of many other comorbidities and risk factors [10]. Indeed, the extent of UA level lowering is not related to the reduction of CV risk [11]. In addition, a recently published study showed no benefit from UA level lowering in patients with coronary artery disease [12]. Moreover, a recent meta-analysis suggested that UA level lowering in patients with heart failure may even be detrimental [13].
The relationship between UA level and blood pressure (BP) is not clear, although most studies suggest BP reduction in patients treated with UA level-lowering agents [2, 3, 14–17]. A newly published network meta-analysis showed that febuxostat caused a statistically significant decrease in diastolic BP; however, no statistically significant effect was found when the authors analyzed the effect of allopurinol, febuxostat, and benzbromarone on systolic BP [18]. On the other hand, Barrientos-Regala et al. found that the extent of UA level lowering was not correlated with changes in BP [19]. A recently published non-randomized study failed to show a significant relation between allopurinol treatment and office or 24-hour BP [20–22]. The relation between UA levels and BP in patients hospitalized in internal medicine wards is unknown.
Aim
The study aimed to evaluate the relationship between UA level and BP among patients hospitalized in an internal medicine department. The second objective was to assess the relationship between UA level and the presence of arterial hypertension. Finally, we intended to investigate the relationship between allopurinol dose and BP.
Material and methods
Hospital records of patients hospitalized in the department of internal medicine from 2016 to 2022 were reviewed. The only criterion of inclusion was a documented level of UA in blood during the hospitalization. Hemodynamically unstable patients (defined as systolic BP below 90 mm Hg or signs of peripheral organ hypoperfusion) were excluded from the study.
Using a standardized data collection form, the data were extracted from the hospital records. The variables considered in multivariable analyses are listed in Table I. There were however differences in medications analyzed in stepwise multivariate analyses with UA level as a dependent variable (Table II) and with BP values as a dependent variable (Table III). In the analysis with UA level as a dependent variable, intake of allopurinol and intake of the following antihypertensive medications were considered due to their potential influence on UA levels: angiotensin-converting enzyme inhibitors, sartans, β-blockers, calcium canal blockers and number of taken diuretics. In the analysis with BP values as a dependent variable, allopurinol and the overall number of antihypertensive agents were considered.
Table I
Baseline characteristics of patients
Table II
Results of the stepwise multivariate analysis with uric acid level as a dependent variable
Table III
Results of the multivariate analysis with blood pressure as a dependent variable
The BP measurements were performed during the morning round in the department by trained professionals using A&D Medical UA-611 BP monitors. The medications the patient was taking during the last 24 h before the measurement, including anti-hypertensive agents, were then accordingly documented. The blood samples were collected by trained professionals from peripheral veins on the day of admission or the first morning of hospitalization. Institutional bioethics committee approval was obtained.
The diagnosis of hypertension was made according to the current guidelines, as were diagnoses of other comorbidities. Body mass index (BMI) was calculated according to the following formula: BMI = weight [kg]/(height [m])2. Normal weight was defined as BMI ≤ 24.99 kg/m2, overweight as 25.0–29.99 kg/m2, stage 1 obesity as 30.0–34.99 kg/m2, stage 2 obesity as 35.0–39.99 kg/m2, and stage 3 obesity as BMI ≥ 40.0 kg/m2. As weight or height were not available in the hospital records in 147 cases, each of these patients was additionally assigned to one of the above-mentioned groups based on the section “diagnoses” in their medical records. Patients whose body type in the section “physical examination” was labeled as “overweight” by the attending doctor, but were not diagnosed with obesity nor had a BMI measurement, were included in the category “overweight”.
The estimated glomerular filtration rate (eGFR) was calculated using the simplified MDRD equation. In 3 cases the eGFR measurements were replaced by a mean value for all individuals due to missing data on the patients’ serum creatinine level. Finally, patients who were included in the category “infection on admission” were either hospitalized primarily for an infection or for a non-infectious condition, which has been aggravated by an acute infection.
Statistical analysis
Continuous variables are presented as medians with first and third quartiles, while categorical values are presented as proportions. The χ2 or the Fisher exact test was applied to all the categorical variables. The Shapiro–Wilk test was used to assess the normality. In the case of non-normal distribution, variables were compared using the Mann-Whitney U test or the Kruskal-Wallis test, as appropriate. The correlations between variables were assessed using Spearman’s rank correlation coefficient. Subsequently, multiple stepwise regression and logistic analyses were performed. In the case of collinearity between two independent variables, the variable which was less significantly related to BP or the diagnosis of hypertension was excluded from the analysis. A two-tailed p-value of less than 0.05 was regarded as indicating statistical significance. The statistics were calculated with Statistica 13 (TIBCO Software, Palo Alto, United States) and MedCalc 20.305 (MedCalc Software, Ostend, Belgium).
Results
Baseline characteristics of patients
Based on the hospital records review, 565 patients were included. We excluded 4 cases from the analysis due to extreme values of UA or BP levels. Ultimately, the data of 561 (297 female and 264 male) patients were analyzed (Table I). The mean age was 65.46 ±17.46 years. Overall, 64.4% of the patients were aged ≥ 60 years (21.8% of patients were aged between 60 and 70 years, 18.9% 70–80 years, and 23.7% were aged ≥ 80 years). The majority of the patients (56.2%) were overweight or had been diagnosed with obesity. The median BMI was 29.3 (24.5–37.0) kg/m2 and the median UA level was 339.0 (261.7–422.3) μmol/l. No patient used a uric acid-lowering drug other than allopurinol. Patients with higher levels of UA had more often been diagnosed with stage 3 obesity, hypertension, heart failure, atrial fibrillation and kidney disease, as well as with an infection on admission to the hospital (Table I). They also had lower eGFR.
Relationship between UA and BP
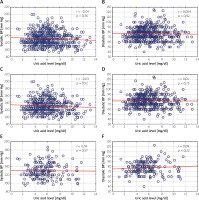
We did not find a significant correlation between the UA level and BP values (Figure 1) when we analyzed the whole group as well as when we limited the analysis to patients who were prescribed no BP-lowering and no UA-lowering drugs. Variables independently related to the UA level are presented in Table II. Sex, obesity and kidney function were consistently related to the UA level, while BP was not independently related to the UA level in any of the studied groups.
Figure 1
Correlations between uric acid level and systolic and diastolic blood pressure (BP). A, B – correlations in all analyzed patients (n = 561), C, D – in patients not taking any uric acid-lowering agents (n = 484), E, F – in patients not taking any uric acid-lowering agents and those not taking any anti-hypertensive agents (n = 189). Spearman’s R coefficient and p-values are shown in the upper right corners
Obesity, BMI, atrial fibrillation and kidney function were related to higher BP levels in univariable analysis (Table IV). Age correlated with diastolic BP (r = –0.36, p < 0.0001), but not with systolic BP (r = –0.06, p = 0.06), while BMI correlated with both systolic (r = 0.22, p < 0.0001) and diastolic (r = 0.36, p < 0.0001) BP. On the other hand, we found no significant correlation between allopurinol dose and systolic BP (r = –0.02, p = 0.57) or diastolic BP (r = –0.04, p = 0.31). Similarly, BP was not correlated with the number of anti-hypertensive drugs used (systolic BP: r = 0.07, p = 0.11; diastolic BP r = –0.08, p = 0.07). Table III presents factors independently related to BP values.
Table IV
Factors related to BP – univariate analysis
Relationship between UA and arterial hypertension diagnosis
Patients with a diagnosis of AH had significantly higher UA levels than patients without AH (median values: 350.9 (270.6–428.3) μmol/l vs. 309.3 (240.9–392.6) μmol/l, p = 0.002). In a group of patients without current uric acid-lowering drug intake (n = 484) UA levels were also higher in patients with AH than in patients without AH (median values: 350.9 (273.6–428.3) μmol/l vs. 309.3 (243.9–392.6) μmol/l, p = 0.003). Similarly, in a group of patients not prescribed any uric acid-lowering and not prescribed any anti-hypertensive drug (n = 189) UA levels were higher in patients with AH than in patients without AH (median values: 362.8 μmol/l (291.5–422.3) vs. 291.5 (237.9–371.8) μmol/l, p = 0.003). Table V presents variables related to the diagnosis of hypertension. Only age, obesity and diabetes, but not UA levels, were independently related to the diagnosis of hypertension.
Table V
Results of logistic analysis with the diagnosis of hypertension as a dependent variable in patients not taking any uric acid-lowering agents. 95% confidence intervals are shown in brackets
Relationship between allopurinol dose and BP
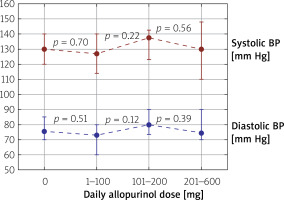
The allopurinol dose was not related to either systolic or diastolic BP (Figure 2). In patients untreated for hypertension allopurinol dose was not independently associated with systolic or diastolic BP (Table III).
Figure 2
Median values of systolic and diastolic blood pressure in all patients included in the study divided into subgroups depending on the daily taken allopurinol dose (0, 1–100, 101–200, 201–600 mg/day). The boundary of the lower whisker is the lower quartile of the data set, and the boundary of the upper whisker is the upper quartile of the data set. P-values are depicted between every two groups
Discussion
The results of our study do not confirm an independent relationship between UA level and BP, or between UA level and presence of AH. Allopurinol dose was not independently related to BP. The outcome is in accordance with several studies, which do not support an independent relationship between UA levels and AH [17, 23, 24].
Among the analyzed patients a significant number had a history of hyperuricemia or hypertension (13.0% and 71.4%, respectively); therefore uric acid-lowering and anti-hypertensive drug intake was also prevalent in the studied group. Individuals with cardiac and renal diseases were included, which has not been the case in many studies concerning this issue. Only 43.85% of the included patients were not overweight or obese. The mentioned numbers highlight the fact that we analyzed primarily the population of overweight and obese patients. Nonetheless, the study group is a representation of multimorbid hospitalized patients. This could account for the fact that the relationship between UA and AH is sometimes difficult to observe in a clinical setting and has been assessed as dependent on other factors such as obesity or diabetes [17].
Many studies have identified age as a factor that substantially impacts the relationship between UA level and BP. Some investigators have demonstrated that the effect of UA on hypertension is larger in younger populations [3, 16, 25]. These findings seem consistent with the hypothesis that prolonged AH is less uric acid-dependent [26]. This is a further factor that could elucidate the lack of an independent relationship in our study, as our study group’s mean age was above 65.
Atrial fibrillation was related to lower systolic BP in our study. The result however should not be seen as contradictory with the well-evidenced causal relationship between AF and hypertension. Firstly, patients during an AF episode are more likely to have lower BP due to an impairment of effective cardiac pump function. In addition, an acute condition may increase heart rate to a greater extent in patients with chronic atrial fibrillation compared to patients with sinus rhythm. This phenomenon is frequently related to decreased stroke volume and subsequently decreased cardiac output and lower BP. This observation is of particular importance as emergency patients constitute around 58% of all patients in our study and they could have had atrial fibrillation on admission, and not only in their medical history. The described results emphasize the impact of a present acute state on the relationship between UA and AH.
Respecting the evaluation of uric acid-lowering treatment and its relation to BP, allopurinol dose was not a factor independently associated with BP in the group of patients without anti-hypertensive drug intake in our study. This is consistent with multiple studies [18–21]. Some meta-analyses regarding this subject conclude the evidence is insufficient to unequivocally confirm the hypotensive effect of uric acid-lowering agents in hypertensive patients [25]. Moreover, age could be a factor limiting the efficacy of this group of agents. In 2001 Mazzali et al. conducted an experimental study on rats to discover a mechanism through which UA could lead to AH [27]. The currently proposed pathomechanism of AH induced by UA is two-staged. Initially, the increase in pressure is caused by vasospasm, while in the second stage, hypertension is probably caused by permanent damage to the endothelium and proliferation of smooth muscles of blood vessels. Some authors conclude that after progression to the uric acid-independent stage of hypertension, uric acid-lowering drugs could become less effective [26]. A more evident preventive and therapeutic effect of those agents in adolescents with prehypertension and hypertension supports this view [28–30]. Concerning our study, we can assume the patients had a longer history of AH and may have been less prone to uric acid-lowering treatment, as the mean age of the studied group was above 65. The size of the analyzed group of patients without anti-hypertensive drug intake however limits the value of this result.
We acknowledge the limitations of our study, one of them being its retrospective nature. Correspondingly, clinical data collection was based entirely on medical records review. Despite BP measurements being conducted by medical professionals, they could have been influenced by multiple factors such as the patient’s emotional state, whose significant impact on the measurements cannot be excluded. A further limitation of our study is the character of the study group. We investigated a hospitalized population of patients; it would be worth comparing and implementing the results of our study in similar groups of hospitalized individuals. The acute conditions which demanded hospitalization were heterogeneous. Presence of an infection and anemia were considered; there were however many other reasons for urgent admission, which could have had a component of rhabdomyolysis and acute kidney injury, and which could have impacted the measurements substantially and unpredictably. The only criterion of inclusion was UA level recorded on admission, hence the presence of individuals with a history of renal, cardiac, metabolic, infectious and oncologic diseases in the study. Indeed, the unselected nature of our study group should be considered an advantage of the present analyses as it mirrors the patients treated in everyday hospital practice.
Conclusions
Our study does not confirm an independent relationship between UA level and BP, nor between UA level and the diagnosis of arterial hypertension in a population of hospitalized patients. Moreover, allopurinol treatment was associated with neither systolic nor diastolic BP. Prospective and interventional studies are warranted to determine the relationship between BP and uric acid-lowering treatment.








