Introduction
Nailfold capillaroscopy is a non-invasive method of visualising the nailfold microvasculature under a stereoscopic microscope or videocapillaroscope. The examination is particularly useful for early diagnosis of systemic scleroderma (SSc), assessment of disease activity and extent of internal organ involvement, as well as for monitoring the effectiveness of the therapy used. The vascular disorders characteristic of SSc are called “scleroderma microangiopathy” and include megacapillaries, reduced numbers of vessels, avascular areas, ramified loops, and microhaemorrhages [1]. Moreover, nailfold capillaroscopy is useful for diagnosis and monitoring of scleroderma-spectrum disorders, such as dermatomyositis (DM), polymyositis (PM), mixed connective tissue disease (MCTD) or undifferentiated connective tissue disease (UCTD) [2]. According to Cutolo et al., the capillaroscopic changes characteristic of systemic sclerosis occurring in scleroderma-spectrum disorders should be defined as scleroderma-like pattern microangiopathy [1]. Such abnormalities are diagnosed in about 20–40% of patients with idiopathic inflammatory myopathies, especially DM accompanied by Raynaud`s phenomenon [3]. The scleroderma-like pattern is found in about 50% of patients with MCTD and in about 5–10% of patients with UCTD [4, 5].
Aim
The aim of the study was to analyse the capillaroscopic pictures in the group of patients affected by scleroderma-spectrum disorders. Furthermore, a possible correlation of capillaroscopic abnormalities with the clinical picture and serological profile was assessed.
Material and methods
The study included 15 patients (13 women and 2 men) with scleroderma-spectrum disorders hospitalized in the Department of Rheumatology and Systemic Connective Tissue Diseases of the Medical University of Lublin in 2021. All patients signed informed consent to participate in the study. The study was approved by the Bioethics Committee of the Medical University of Lublin. In the study group, 7 patients met the classification criteria of the European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) of 2017 [6] (Table 1) and 8 patients were diagnosed with MCTD based on the Alarcon-Segovia diagnostic criteria [7] (Table 1). In the group of patients with DM and MCTD, the incidence of the selected clinical symptoms (Raynaud’s phenomenon, arthritis, oesophageal involvement, interstitial lung disease, muscle involvement, heart involvement) and the presence of antinuclear antibodies (ANAs) was analysed (Table 2). All patients underwent nailfold capillaroscopy using a Dino-Lite USB-microscope with 200× magnification. The first row of nailfold capillaries was examined in fingers 2–5 of the right and left hand. Fifteen minutes prior to the examination, the patients were advised to stay at room temperature to prevent Raynaud’s attacks. Immersion oil was used to improve the skin translucency. The nailfold microcirculation was assessed qualitatively according to Cutolo dividing the images into normal, non-specific lesions, and scleroderma-like microangiopathy [8]. The non-specific changes included the presence of twisted capillaries (over 40%), microhaemorrhages, and dilated loops with diameters ranging between 30 μm and 50 μm. The numb 30.06.2024. er of capillaries, the presence of dilated, giant and ramified capillaries as well as derangement of vascular loops were evaluated. The capillaries with a diameter of more than 50 μm were defined as giant, and the number of capillaries below 7/mm indicated a reduction. The severity of abnormalities was considered mild when the number of vascular disorders was ˃ 33%, moderately numerous – 33–66% and numerous – above 66%. According to the classification of Cutolo et al., the pattern of microangiopathic changes was defined as early, active and late [9].
Table 1
Characteristics of the study group
Table 2
Clinical characteristics of the DM and MCTD groups
Results
In the study group of 15 patients, 7 were diagnosed with DM and 8 with MCTD. Scleroderma-like microangiopathy was found in 7/15 (47%) patients with scleroderma-spectrum disorders, including 3/7 (43%) with DM and 4/8 (50%) with MCTD. The patterns of vascular abnormalities found in the group with scleroderma-like microangiopathy were as follows: early in 3 (42%) patients with MCTD (42%), active in 2 (29%) patients (1 with DM and 1 with MCTD) and late in 2 (29%) patients. The early pattern was observed only in the group with MCTD and the late pattern only in patients with DM.
The non-specific changes were found in 4/15 (27%) patients, including 2/7 (28%) with DM and 2/8 (25%) with MCTD. Normal capillaroscopic images were observed in 4/15 (27%) patients, including 2/7 (28%) with DM and 2/8 (25%) with MCTD. The presence of dilated, giant and ramified capillaries, microhaemorrhages, reduced numbers of capillaries, avascular areas and derangement of vascular loops in DM and MCTD are presented in Table 3. The dilated, giant and ramified vessels were observed more often in DM, as compared to MCTD, but these changes were not statistically significant.
Table 3
Comparison of capillaroscopic changes in DM and MCTD
Analysis of correlations with selected clinical symptoms in the group of patients with scleroderma-spectrum disorders demonstrated that a reduced number of vessels was statistically significantly more common in patients with interstitial lung disease (Table 4). There was no correlation of capillary loss with other clinical and serological symptoms of DM and MCTD. Moreover, no other significant correlations between capillaroscopic changes and the clinical pictures were found.
Table 4
Correlation between capillary loss and interstitial lung disease in scleroderma – spectrum disorders
| ILD vs. number of capillaries | P = 0.0350 | Yule’s Φ = 0.5584 |
The comparison of DM and MCTD groups revealed that the incidence of the Reynaud’s phenomenon and arthritis was statistically significantly higher in patients with MCTD, while the levels of muscle enzymes were statistically significantly higher in DM.
As regards the serological profile of the study group, antinuclear antibodies (ANAs) were found in 15 (100%) patients and extractable nuclear antigens (ENAs) in 13/15 patients, including 5/7 with DM (1 – anti-Jo1, 1 – anti-Mi2, 2 – anti-SS-A, 1 – anti-Ku); anti-RNA antibodies were detected in all patients with MCTD.
All patients were treated with hydroxychloroquine and glucocorticoids; 4 patients received cyclophosphamide i.v., another 4 – mycophenolate mofetil p.o. and 3 – methotrexate s.c.
Discussion
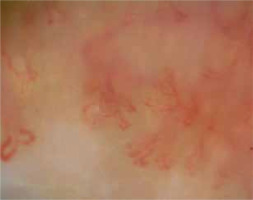
Scleroderma-like microangiopathy is found in about 40–50% of cases of scleroderma-spectrum disorders [10]. It is observed in about 20–40% of patients with idiopathic inflammatory myopathies, more often in DM than in PM, especially when accompanied by the Raynaud’s phenomenon. Manfredi et al. have suggested that capillaroscopic changes typical of microangiopathy are associated only with DM and not with MCTD [11]. Characteristic vascular abnormalities in DM are neoangiogenesis (bushy, dendriform vessels), moderate disorganization of the vascular system with a relatively low number of avascular areas. Moreover, bushy and dilated vessels predominate (Figure 1) [3]. Considering significant differences in capillaroscopic images of SSc patients compared to DM patients, Mugi et al. have implied that in DM the term “microangiopathy in the course of DM” should be used instead of “scleroderma-like microangiopathy” [12]. Furthermore, Sebastiani et al. have demonstrated capillaroscopic abnormalities in 62% of patients with antisynthetase syndrome; the pattern of scleroderma-like microangiopathy has been found to correlate with the presence of anti-Jo1 antibodies, yet not with the Raynaud’s phenomenon [13]. On the other hand, more than 40% of patients with idiopathic inflammatory myopathies have normal capillaroscopic images, and some have nonspecific changes [10].
According to the literature data on MCTD, the scleroderma-like capillaroscopic changes are found in about 50% of MCTD patients; in 20% the changes are non-specific while in 30% the images are normal. The early pattern of scleroderma-like microangiopathy predominates (Figure 2) [10]. In their retrospective assessment of capillaroscopic images in patients with MCTD, UCTD and primary Raynaud’s phenomenon, Pizzorni et al. have demonstrated that the incidence of scleroderma-like microangiopathic capillaroscopic changes was statistically significantly higher in the group of patients with MCTD, as compared to UCTD [5]. Moreover, the presence of neoangiogenesis, giant capillaries and capillary reduction was more pronounced in MCTD than in UCTD. The authors have concluded that the capillaroscopic abnormalities in UCTD resemble the images observed in the primary Raynaud’s phenomenon (nonspecific changes), as opposed to MCTD in which scleroderma-like microangiopathic changes prevail.
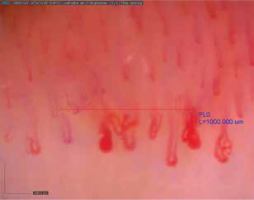
Figure 2
Capillaroscopic changes in mixed connective tissue disease (scleroderma-like pattern microangiopathy)
Our observations on the incidence of capillaroscopic abnormalities are similar to the literature data. The scleroderma-like microangiopathic changes were found in approx. 50% of patients, including about 40% with MD and 50% with MCTD. The non-specific changes were observed in about 30% of patients with DM and in 25% with MCTD. About 30% of patients with DM and 25% with MCTD had normal capillaroscopic images. Interestingly, the dilated, giant and ramified vessels were found to be more common in DM compared to MCTD; however, these changes were not statistically significant. The observations of various authors reveal that neoangiogenesis also predominates in patients with DM [3, 11, 12]. In our study, the incidence of Raynaud’s phenomenon and arthritis was statistically significantly higher in patients with MCTD compared to DM while the level of muscle enzymes was statistically significantly higher in DM. However, there was no correlation of individual clinical symptoms with capillaroscopic vascular abnormalities and scleroderma-like pattern microangiopathy. The analysis of individual patterns of scleroderma-like microangiopathy showed that the early pattern occurred only in the group of patients with MCTD, which is consistent with the literature data [10, 14].
The literature reports have demonstrated the correlations of capillaroscopic changes with clinical and serological symptoms as well as the degree of internal organ involvement in scleroderma-spectrum disorders [10, 14–18]. A correlation of scleroderma-like microangiopathy with a higher concentration of creatine kinase and the severity of skin lesions has been found in DM [12]. Moreover, Barth et al. have shown a correlation between the number of vessels and lung involvement in patients with juvenile DM [15]. In the group of patients with linear capillary density below 6 capillaries/mm, lower percentages of forced vital capacity, total lung capacity and carbon monoxide diffusion capacity have been observed, as compared to the group of patients with normal numbers of vessels [15]. An association of scleroderma-like microangiopathy, particularly reduced capillary numbers, with interstitial lung disease has also been demonstrated in MCTD [16, 19]. Our findings confirm the literature data; in the study group of patients with scleroderma-spectrum disorders, the incidence of reduced numbers of vessels was statistically significantly higher in patients with interstitial lung disease.
The limitation of our study is small groups of patients. Furthermore, no analysis of patients with polymyositis or undifferentiated connective tissue disease was performed. The research will be continued in larger samples of patients with other scleroderma-spectrum disorders.
Conclusions
Scleroderma-like microangiopathy is quite common in scleroderma-spectrum disorders; hence, nailfold capillaroscopy is a valuable tool helping to diagnose and assess the activity of these disorders [20, 21]. A significant association of capillary reduction with severe organ complications, such as interstitial lung disease, has been demonstrated in dermatomyositis and mixed connective tissue disease. Therefore, the assessment of capillary density is important for monitoring scleroderma-spectrum disorders, and a quick vascular loss is associated with their higher severity.
Long-term assessment of capillaroscopic changes can be of significance for therapeutic decision making, changing the treatment used and monitoring patients with systemic scleroderma-spectrum disorders.