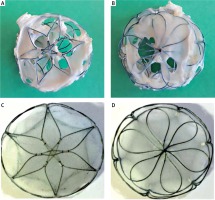
Percutaneous closure of ostium secundum atrial septal defects (ASD II) is the contemporary treatment of choice. It has proven to be effective and safe, with very low complication risk. We present two patients: a 9-year-old boy with an 18-mm-diameter ASD II closed using the 20-mm Cardia Ultrasept occluder and a 6-year-old girl with a 16-mm-diameter ASD II closed using the 20-mm Ultrasept occluder without any periprocedural complications. The positioning of the devices was correct and complete closure of the shunts was confirmed in the follow-up echocardiography. Patients were in routine follow-up and echocardiographic examination 4 and 3 years after implantation respectively. The follow-up revealed a few streams of left-to-right shunts through the device. The shunts were hemodynamically significant, causing volume overload and right atrial and right ventricular enlargement. Consequently, the devices were surgically removed and the ASDs were closed with pericardial patches 6 and 4.5 years after implantation respectively. Visual inspection revealed multiple perforations in the left and right parts of both devices – only nitinol struts with small parts of the devices were covered with endothelium (Figures 1 A, B). The Ultrasept occluder is intended for closure of interatrial communications. It consists of two discs connected with a self-centering waist made of a nitinol wire frame and covered with an Ivalon (polyvinyl alcohol – PVA) membrane (Figures 1 C, D). PVA is a synthetic polymer commonly used in medical devices due to its biocompatibility and high water solubility [1]. The Ultrasept occluder is a well-proven implant with good midterm results. In our patients, complete closure of the ASDs was observed in follow-up echocardiography 3 and 4 years after implantation with the next examination revealing significant shunts. It was probably due to incomplete endothelization of the device prior to the material’s absorption by the tissue. Similar dissolution of the PVA membrane has previously been reported as early as 1 week and as late as 2 years after implantation. Both options for surgical removal of the damaged device and surgical closure of the defect as well as percutaneous closure of leaks with the new device were used [2, 3]. Also, the nitinol wire frame fracture was observed in the similar Cardia Ultrasept device. Because of the risk of late or very late complications after percutaneous ASD closure patients require regular and life-long follow-up care. Any complication related to the device should be reported and analyzed to facilitate implant modification and constructional changes. The Cardia Ultrasept II with interposed Gore-Tex patch is the modified last generation of Cardia devices. The new device was demonstrated to be safe and feasible. With the interposition of the Gore-Tex, it has been possible to abolish perforation of the Ivalon membrane as a complication. Such conclusions can be drawn from a study on 30 Mexican patients after atrial septal defect closure with no incidence of perforation at follow-up for 6 (range: 1–15) months [4].