Introduction
Port-wine stain (PWS) are a common type of cutaneous capillary malformations that affect 0.3% of newborns. They are related to segmental mosaicism and they can occur in all body regions following the patterns of vascular embryogenesis [1–4]. Lasers are the first line option for the treatment of PWS. There are several types of devices available for PWS treatment, including pulse-dye laser (PDL), intense pulse light (IPL), small and large spot 532 nm KTP (potassium-titanyl-phosphate) laser, and 1064 nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser. Others such as alexandrite 755 nm or diode 800–983 nm lasers are less commonly used and argon, copper and krypton lasers are currently not recommended because of the poor safety-to-efficacy profile [5]. Recently photodynamic therapy (PDT) has shown promising results for the treatment of PWS in Chinese patients [6].
Beside PDT, all these devices use hemoglobin as a photon absorber to destroy affected vessels. The majority of patients respond to treatment, but clearance is hardly ever achieved. The degree of improvement depends on several factors including the type of device used and PWS localization. Although PDL is recognized as the most effective, the large spot 532 nm KTP laser has recently been shown to be highly valuable for facial PWS. This device is based on a frequency-doubled, 532 nm Nd:YAG laser and is characterized by a large spot (up to 12 mm), short pulse and contact cooling. Earlier reports on 532 nm KTP lasers were performed with tools that generate a small spot size with up to 2–4 mm diameter and/or long pulse width [7–10]. Larger diameter of the laser beam is crucial for the green light (532 nm) to sufficiently penetrate deep into the dermis or even the subcutaneous fat so as to close the dilatated vessel, as was shown in both vivo and in vitro studies [11, 12].
The assessment of the efficacy of PWS treatment is usually based on the subjective methods of physicians or the patient’s judgment. This makes comparison of the results of different studies difficult. Several objective methods of analysis of two-dimensional (2D) digital or digitalized photography were proposed, but they do not consider the complex shape three-dimensional (3D) nature of most PWS. Other devices based on colorimetric methods assess only the color of the lesion, neglecting its area [13–17]. Analyzing combined measurement of an average color of the lesion and area measured with 3D image analysis has proven its value in our previous reports on facial PWS [5, 18]. In these reports we used a commercially available tool for taking 3D face and body images. Here we propose to use this tool for the objective assessment of PWS located on the neck and trunk.
Aim
The aim of the study was to assess the efficacy of the large spot 532 nm laser in the treatment of neck and truncal PWS using both 3D color and area analysis and subjective judgment of improvement made by the physician.
Material and methods
The study was approved by the Institutional Review Board. We performed a one-center, retrospective study, which included patients with both previously treated (15) and untreated (5) PWS who came to our clinic between January 2014 and June 2018 and who were treated by two physicians (B.K. and M.R.). The PDL was previously used on 4 of these patients, IPL also on four, large spot KTP on 3 patients, and small spot KTP and argon laser were both used on 1 patient. Three patients were treated with an unknown laser. Only patients who had 3D photographs performed before and after and had at least one single procedure were included in the study. All patients were Caucasians (13 females and 5 males), aged from 12 to 66 (mean ± SD: 37 ±14.3). Most patients (13) had skin phototype II according to the Fitzpatrick scale and 1 had phototype I and 4 patients had phototype III. Patients currently tanned or with a history of sun and/or ultraviolet exposure within 1 month prior to the procedure were asked to come after more than 4 weeks of careful sun protection. All patients were treated with a large spot, frequency-doubled, 532 nm Nd:YAG (KTP) laser with 5ºC contact cooling provided by sapphire glass (ExcelV; Cutera Inc, Brisbane, CA, USA). A variable setting was used with the fluencies ranging from 7 to 9 J/cm2, pulse duration ranging from 6 to 9 ms and spot size ranging from 6 to 9 mm according to the judgment of the physician. The largest available spot size for the preset fluence and time was preferable. Local anesthetic (tetracaine and lidocaine ointment for 30 min prior to the procedure) was used in 1 adolescent patient. Cooling with a cold pack was used for 20–30 min after each procedure. Patients were asked to use a post-treatment emollient for 7 days, avoid sun exposure and use topical preparations with sun protection factor 50+. The minimal interval between treatments was 4 weeks.
3D images were taken with the Vectra XT (Canfield Scientific, NJ, USA) in standardized conditions according to the manufacturer’s guidelines for facial and truncal images and analyzed as described previously [18]. Thirteen neck and seven truncal lesions were assessed. Selected surface area (cm2) and selected area average color (described with L*a*b* coordinates) were used for further analysis. Whenever possible, healthy skin of a symmetric area served as a control for color evaluation. In other cases (e.g. lesion covering two sides of the neck), skin adjacent to the lesion was used. The difference between the color of the lesion and healthy skin (ΔT) was calculated according to the following equation [14, 16]:
To establish the improvement of the color of the lesion after treatment, the clearance effect (CE) was calculated as follows:
Reduction of the area (A%) of the lesion was calculated as the percentage difference between the area (A) before and after the treatment:
Finally, to combine the A% with the CE the, global CE (GCE) was calculated as follows:
Maximal GCE observed throughout the treatment of the patient was defined as GCEmax. The rates of the patients achieving GCEmax of minimum 25% (GCE 25), 50% (GCE 50), 75% (GCE 75) and 90% (GCE 90) during the treatment were calculated (Table 1). For safety evaluation, patients were asked to report the presence and longevity of erythema, edema and bruises as well as the occurrence of blistering and/or crusting or secondary infections. Skin was assessed for the presence of scars on each visit. Subjective evaluation was performed using flattened (3D into 2D) images from 0, 45, and 90°. A five-grade scale of improvement was used: 0 (no improvement ), 1–25% (mild), 26–50% (moderate), 51–75% (significant), 76–100% (cured) (Table 1). For some comparisons with objective analysis, an additional threshold point of subjectively assessed ≥ 90% improvement was used. Subjective analysis was performed by two physicians independently (JS, MR). Whenever there were discrepancies between their grading, the images were reevaluated until a consensus was achieved. This happened in two out of 20 cases.
Table 1
Comparison of two systems of PWS treatment evaluation
Statistical analysis
Statistical analysis was performed with Statistica 12.0 software (StatSoft, USA). Quantitative variables were characterized with mean, standard deviation or median, quartiles and range after testing normality with the Shapiro-Wilk test. Significance of differences between two groups of variables was tested with the Mann-Whitney test. Student’s t-test was used for comparison between two related groups. Correlations were calculated with Spearman’s test. Intergroup comparisons of discrete variable distributions were carried out with Pearson’s χ2 test. All p-values < 0.05 were considered to be statistically significant.
Results
Efficacy
Median number of treatment sessions to obtain a maximal response was 6 (range: 2–21) (Figure 1 A). Mean maximal improvement achieved during the treatment assessed objectively (GCEmax) was 57% (n = 20) (Figure 1 B). Median GCEmax in previously untreated patients was higher (69.9%) than in previously treated patients (55.5%) (Figure 2 A). There was no significant difference in the response between truncal PWS and those located on the neck (Figure 2 B). Objectively assessed improvement (GCEmax) significantly correlated with subjective grades (Figure 3 A). To further analyze the correlation between these two methods, we used a response rate analysis employing objectively measured GCE 25, GCE 50, GCE 75, GCE 90 percentage response rates and similar rates calculated from subjective analysis (Figure 4). Improvement of minimum 25% (GCE 25) was achieved by all patients but 1 (95%), and by all patients in subjective analysis. Fifty percentage response rate was achieved by 75% and 70% of patients in objective (GCE 50) and subjective methods. The biggest differences were seen in the rates of 75% and 90% improvement assessed with the two methods. Only 15% of patients achieved GCE 75, compared to 60% of patients with this level of improvement in subjective evaluation. There was a statistically significant correlation between these two methods (Figure 3 B).
Figure 1
A – Number of laser sessions per patient. B – Maximal improvement (%) achieved in 20 patients defined as maximal global clearance effect (GCEmax). C–E – Before and after images (flattened 3D images) of three different PWS that were rated as 75–100% improvement (‘cured’ grade) subjectively. However, they are differently classified with the objective 3D measurement of the area and color. C – Objective result of the treatment (GCEmax) of the PWS located on the chest (trunk) is 88.0%. D – GCEmax of the PWS located on the back is 66.6%. E – GCEmax of the PWS located on the neck is 50.7%
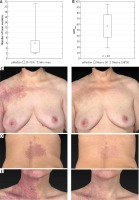
Figure 2
A – Comparison of maximal improvement defined as maximal global clearance effect (GCEmax) between patients previously untreated and previously treated. B – Comparison of GCEmax between PWS located on trunk and neck
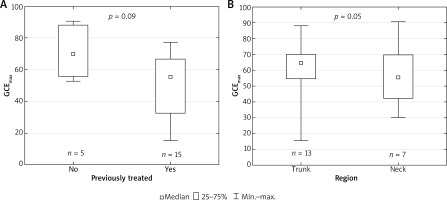
Figure 3
Correlations between objective and subjective methods of improvement assessment. A – Statistically significant positive correlation between GCEmax values and grade of improvement based on subjective evaluation. Additional rate of ≥ 90% was added to commonly used thresholds of 25, 50 and 75% for the subjective evaluation. B – Positive correlation between the percentage grade of improvement derived from objective analysis and the percentage rates of improvement based on the subjective evaluation. Relatively low presence of a ‘50–74%’ grade is seen in the subjective analysis

Figure 4
Black bars represent percentage of patients who have achieved maximal improvement of ≥ 25% (global clearance effect (GCE) 25), ≥ 50% (GCE 50), ≥ 75% (GCE 75) and ≥ 90% (GCE 90) in objective 3D image analysis. Corresponding white bars represent the percentage of patients who have achieved improvement of similar rates assessed in subjective analysis performed by physician (n = 20)

Because most studies on treatment efficacy do not use percentage rates of improvement with 25, 50 and 75% thresholds, but grades of improvement based on ranges, we compared objective and subjective methods using a similar approach (Table 2). There was no statistically significant difference between the two methods, but this analysis reveals a difference between the two types of analysis at the grade of ‘significant’ (51–75%) improvement.
Safety
All patients experienced minor dermal edema lasting usually for up to 4 days and not lasting longer than 7 days. Bruising was also inevitably present and lasted for 7–14 days. Crusting and/or blistering was present only focally in a minority of patients. No new scarring was observed except for pre-existing scars after previous procedures in 4 patients. No secondary skin infections were observed.
Discussion
Our study proved that the large spot 532 nm laser is effective in the treatment of neck and trunk PWS in Caucasian patients (Figures 1 C–E). Objectively measured rates of improvement were similar to those found for facial PWS [5, 18]. In these earlier studies we found that in previously untreated facial PWS median GCEmax was 70.4%, which is similar to 69.9% found in the current study. Similarly, for previously treated patients with facial PWS median GCEmax was 59.09%, which resembles 55.5% found for neck and truncal PWS in the present study. We found no difference in GCEmax between neck and truncal lesions (Figure 2 B). Median number of laser sessions required to gain GCEmax in our patients was 6, and this is also comparable to 7 sessions in patients with facial PWS. Thus, all axially localized PWS respond to the large spot 532 nm laser in the same way. The results obtained here indicate that this method can be judged as a first line treatment not only for facial, but also for neck and truncal PWS together with PDL lasers for patients with fair skin. However, direct comparison in a prospective study would be conclusive.
We introduced the 3D method of PWS color and area evaluation to increase both the accuracy and the objectiveness of treatment outcome measurements. New devices such as the large spot KTP laser presented here and others, e.g. the dual 590/1064 nm laser, PDT with hemoporfin intravenous injection and new medications are being tested for treatment of PWS. Most of the previous and current studies are based on the subjective visual judgment performed by the physician. The common scale used in these reports has a higher threshold point set at 75%. With the median GCEmax of about 70% achieved in untreated patients with the large spot KTP laser, this scale seems to be inadequate. We have proved here that objective assessment proposed by us correlates with commonly used subjective methods. However, there are some clear differences. There is a tendency to overestimate the improvement rate performing subjective evaluation in the group of patients who respond with more than 75% improvement (Figures 1 E, 3, 4, Table 2). It was shown previously that objective analysis of the efficacy of treatment of CM gives slightly worse results than subjective methods [15]. This also supports the use of the new 3D objective analysis for the evaluation of modern highly efficient treatment modalities. Interestingly, there seems to be an opposite phenomenon in the group of patients who responded with less than 50% improvement (Figures 3, 4, Table 2). There were 3 patients who were judged objectively as improved by more that 50% but 25–49% in the subjective method. In general, results obtained in subjective methods are split into 25–49% improvement and ≥ 75 with only 10% judged as 50–75% improvement (Figure 3, Table 2). In contrast, the distribution of results for the objective method follows a normal distribution pattern with the most common GCEmax values being about the mean (Figure 3 A).
We believe that for the future studies it is better to present results as a percentage rate of improvement rather than as grades. In our previous report we introduced GCE 25, GCE 50, GCE 75 and GCE 90 rates [18]. Physicians and authorities are acquainted with the PASI (Psoriasis Area And Severity Index) 50, PASI 75, PASI 90 terms, and they are currently the main endpoints for the evaluation and comparison of different psoriasis treatment modalities. Results presented in this fashion are clearer than grades. For example, in our study only 10 patients had a significant (51–75%) response in subjective evaluation (Table 2), but 70% of patients had more than a 50% response (Figure 4). The latter better reflects the real situation and provides data that can be easily compared with other studies.
We have found the large spot, 532 nm laser treatment with contact cooling to be safe for treatment of the neck and trunk. All side effects were transient and mild.
Conclusions
The results of our study indicate that the large spot 532 nm laser is an effective option for the treatment of neck and truncal PWS and can be used as a first line regimen in patients with type I–III skin phototypes. Objective assessment of the efficacy with 3D image analysis of the neck and trunk is an accurate and reliable method and can be used in future studies to directly compare different methods of PWS treatment.








