Introduction
Basal cell carcinoma (BCC) is the most often diagnosed cancer in the fair-skinned population with constantly increasing prevalence [1]. Although it has a generally slow growth pattern, lesions located on the face appear to be more aggressive [2, 3]. BCC occurs about four times more likely in the region corresponding to embryonic fusion planes (EFP) [4]. This region called the “H-zone” consists of the nose, eyes and ears, and has been associated with a higher risk of deeper tissue invasion [4, 5].
Although dermoscopy can increase accuracy of diagnosis of BCC up to 90%, dermoscopic criteria of BCC may be less effective especially for non-pigmented variants of BCC, therefore they undergo continuous assessment and updates, when necessary [6].
Aim
The purpose of the study was to describe the dermoscopic characteristics of vessels of BCC in the H-zone and non-H-zone which may most appropriately characterize those two locations.
Material and methods
Retrospective examination of dermoscopic images of BCCs from 120 patients with skin phototype II was performed. Patients with genetic disorders predisposing to BCC were excluded from the analysis. Dermoscopic evaluation with a light pressure and solely with ultrasound gel application was performed with Medicam 1000 (FotoFinder Systems GmbH) under 20× magnification by three independent, experienced observers. The anatomic location of tumours was classified as the H-zone (nose, ears, eyes) and non-H-zone (forehead, cheeks, chin and the rest of the face and neck). If the lesion had been located in the border zone between the aforementioned anatomical locations, its eventual classification was based on the discussion of all three authors.
Several types of vessels, namely arborizing telangiectasias, short fine telangiectasias (SFT), glomerular, comma, loop, linear irregular and dotted vessels were described. All types of vessels were classified as present or absent and then comparison between the H- and non-H-zone was made.
Statistical analysis
The categorical variables were presented in both absolute numbers and percentages, while due to non-normal distribution assessed with the Shapiro-Wilk test for the continuous variables, their results were summarized using the median with quartile 1 and 3. Between-group comparisons of continuous variables were conducted using the Mann-Whitney U test, while Pearson’s χ2 test was used to evaluate the categorical variables. The interval of two-sided p < 0.05 was considered statistically significant. Statistica 10 (StatSoft Inc., Tulsa, OK, USA) was used for all calculations. The approval of the bioethics committee was not required, based on the PCN/0022/KB/34/21 decision of the Bioethics Committee of the Medical University of Silesia in Katowice.
Results
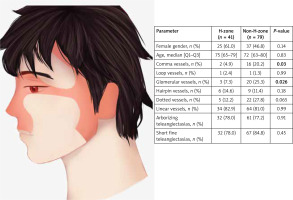
Of the 120 lesions analysed, there were 41 (34.2%) in the H-zone and 79 (65.8%) in the non-H-zone as presented in Figure 1. The vascular pattern differed, depending on whether the lesion was located in the H- or non-H-zone, however the two main vascular structures in BCC – arborizing vessels (78.0% and 77.2% in the H-zone and non-H-zone) and short-fine-telangiectasias (78.0% and 84.8% in the H-zone and non-H-zone) did not differ significantly as reported in Table 1. The histological types of tumours are presented in Table 2. Significant differences were observed for the occurrence of glomerular and comma vessels. Both occurred less frequently in the H-zone (7.3% for glomerular and 4.9% for comma vessels, respectively) than in the non-H-zone (25.3% for glomerular and 20.2% for comma vessels). There were no further significant differences in the prevalence of other vessels than noted above.
Table 1
Vascular pattern in dermoscopic images of BCC in the H- and non-H-zone
Table 2
The comparison of the histological subtypes in the H- and non-H-zone
Discussion
Vessels are known to be essential for the tumour growth and increased microvascular density in BCC was observed [7]. Because the significance of embryological processes in aetiology of BCC is still under discussion, detailed analysis of the vascular patterns and their correlation with regions of fusion of embryonic masses (H-zone) may provide new data to the aforementioned relationship and the clinical course of skin cancer. To the best of our knowledge, there are no reported data describing the vascular pattern in dermoscopy of BCC in the H- and non-H-zones.
In our study, arborizing telangiectasias and short-fine telangiectasias were the most frequently seen types of vessels in both face regions, which is consistent with the literature [3, 6, 8]. Compliance of our results with the literature in terms of two main vascular structures can confirm that our study was conducted according to the generally accepted recommendations [2]. Furthermore, no difference in the presence of both types of vessels between the H- and non-H-zones allows us to assume that their occurrence is independent of the anatomical location of BCC.
Among other types of analysed vessels, namely, loop vessels, dotted vessels and linear vessels, there were no differences between the H-zone and non-H-zone. However, significant differences were observed as far as the glomerular and comma vessels were concerned. It should be emphasised that we recorded a surprisingly high percentage of comma vessels in the non-H-zone, although there are some reports in the literature confirming their presence even up to 45% [9].
Glomerular vessels, typical for squamous cell carcinoma, can also be present in BCC, although such high percentages as in our study have not been reported so far. As the detection of vessels in dermoscopy is often instrument-dependent, it should be noted that our study has been conducted using the same high-resolution device, namely Medicam 1000 (FotoFinder Systems GmbH), which provides high image quality, what may have determined the higher detection of the aforementioned vessels [6, 8].
Moreover, we have noted a significant difference for histological characteristics, including more superficial varieties for the non-H-zone. According to the literature, superficial BCCs can be characterized by a higher percentage of comma and glomerular vessels [9] and this may perhaps explain the predominance of comma and glomerular vessels in the non-H-zone.
Finally, it could be speculated that the differences between aforementioned two types of vascular structures of BCCs present in H- and non-H-zones, which at the current state of knowledge are of uncertain significance, may gain further importance with the increasing use of high-resolution equipment and higher magnifications, including the use of optical super-high magnification dermoscopy which could shed additional light on the dermoscopic diagnostics in BCC and may allow to redefine vascular criteria in BCC [10].
Conclusions
The dermoscopic morphology of the vessels in BCC tumours in the H- and non-H-zones is generally similar, with differences in the presence of glomerular and comma vessels. As the role of embryology and anatomic location in the pathogenesis of BCC is still under discussion, further studies focused on the vascular structures of these malignancies in those two locations are needed to verify the clinical significance of our findings.