Introduction
Vitamin B6 is a water-soluble chemical compound, naturally occurring in six different forms: pyridoxine (PN), pyridoxine 5’-phosphate (PNP), pyridoxal (PL), pyridoxal 5’-phosphate (PLP), pyridoxamine (PM), and pyridoxamine 5’-phosphate (PMP). The most important biologically active form of vitamin B6 is PLP, which acts as a coenzyme in over 140 biochemical reactions including carboxylation, replacement reactions, transamination, aldol cleavages, deamination, and racemisation [1]. Therefore, vitamin B6 is involved in various biological processes, such as amino acid and homocysteine metabolism, glucose and lipid metabolism, DNA/RNA synthesis, and modulation of gene expression [2]. It also factors in the synthesis of neurotransmitters; it converts levodopa into dopamine and facilitates the conversion of glutamate to GABA [3], and also affects immune cell function and blood-forming processes [4, 5]. Vitamin B6 is absorbed in the jejunum, and it is metabolised to its active form in the liver. Excess vitamin B6 is eliminated through kidneys (with the half-life elimination of 15-20 days) [6]. Vitamin B6 deficiency causes dermatitis and glossitis [7], disrupts function of the nervous system (depression, confusion, neuropathy) [8] and immunity [9], and promotes cancer development and progression [10]. Decreased levels of vitamin B6 in the body are found in a number of pathological conditions, such as chronic alcohol dependence, obesity, inflammatory bowel disease, chronic renal failure, and rheumatoid arthritis [11-13].
The level of vitamin B is severely reduced in protein malnutrition. The main symptoms of protein deficiency are reduced body and muscle weight and decreased protein concentration in the serum [14]. Prolonged protein malnutrition decreases levels of hormones, cytokines, and growth factors and results in various organ dysfunctions (especially liver and skin), hypoalbuminaemia, vascular damage, and hepatic steatosis [15-17]. It also affects haematological and immunological parameters [18]. Studies on BALB/c mice fed a protein-deficient diet (20 γ of casein/kg diet) showed that the protein malnutrition affects proliferation of splenic cells and maturation and function of T lymphocytes, and causes changes in lymphoid organs significantly affecting the immune and inflammatory responses [19-21].
Our previous research showed that vitamin B6 supplementation reduces negative effects of protein malnutrition, improves body weight, and positively affects haemoglobin parameters in rat blood [18, 22]. Here we evaluated the effects of moderate, long-term (ninety days) exercise on selected haematological, immunological, and biochemical parameters in rats fed a normal diet, a protein-deficient diet, and a protein-deficient diet supplemented with vitamin B6.
Material and methods
Animals
All animal experiments were conducted according to the Polish regulations and standards of the welfare of laboratory animals. All experiments were accepted by, and conducted according to, the ethical guidance of IV Warsaw Local Bioethics Committee.
The study was performed on 60 male Wistar rats with a body mass of 130 ±7 γ (mean ±SD). The animals were housed in cages (five per cage) in an air-conditioned room under standard conditions (12-hour light cycle, temperature 23oC). The rats were fed ad libitum for 90 days; their diet was semi-synthetic with the energy value of 350 kcal/100 γ (1466.5 kJ/100 g). The rats were divided into six groups according to diet type and exercise: a group with a control diet (10 rats), a protein deficient diet (10 rats), a protein deficient diet + vitamin B6 (10 rats), a control diet + exercise (10 rats), a protein deficient diet + exercise (10 rats), and a protein deficient diet + vitamin B6 + exercise (10 rats). The rats were exercised five days per week. The training consisted of a one-hour run on a treadmill at a speed of 20 m/min. The animals and the food containers were weighed twice a week, and the average body weight and food consumption were calculated.
Diets
We used three types of diet:
Control diet – 20% of energy derived from protein, 65% from carbohydrates, and 15% from fat, of which approximately 2% were essential fatty acids (EFAs).
Experimental diet – 4.5% of energy derived from protein, 80.5 % from carbohydrates, and 15% from fat, of which approximately 2% were EFAs.
Experimental diet – 4.5% of energy derived from protein, 80.5% from carbohydrates, and 15% from fat, of which approximately 2% were EFAs, supplemented with vitamin B6 (300% of the norm).
The disparity in carbohydrate content between different diet groups resulted from the standardisation of the calorie content. The diets were supplemented with minerals and vitamins, in accordance with guidelines for rats. Detailed composition of the diets was described previously [22].
Blood and organ isolation
Rats were anesthetised (intraperitoneal injection of ketamine [120 mg/kg] and xylazine [12 mg/kg]; Polypharm S.A., Warsaw, Poland) and blood was collected in test tubes containing EDTA (for further haematological and immunological analyses) or “on clot” (serum, for further biochemical analysis). After blood collection the rats were sacrificed by an anaesthetic overdose (pentobarbital, 400 mg/kg; Polypharm S.A.) and selected organs (liver, kidneys, spleen, lungs, heart, testicles, and muscle [soleus]) were isolated and weighed.
Haematological analysis
Blood (50 μl from EDTA tubes) was analysed in a haematological analyser (Exigo veterinary haematological system; Boule Medical AB, Stockholm, Sweden). The following parameters were evaluated: white blood cells (WBC), red blood cells (RBC), haemoglobin (HGB), haematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), and platelets (PLT). The results are presented as the mean ± standard error.
Immunological analysis
Determination of WBC phenotypes was performed by flow cytometry (FACS Calibur; BD Biosciences). The following test were used: Rat T/B/NK Antibody Cocktail with Isotype Control (anti-Rat CD3 APC, anti-Rat CD45RA FITC, anti-Rat CD161 PE, anti-Rat CD3 APC, anti-Rat CD8a FIT, anti-Rat CD4 PE [all from BD Biosciences, Warsaw, Poland]). 50 μl of blood from EDTA-containing tubes was used for the analyses. The samples were incubated with antibodies for 20 min, centrifuged (500 × g, 5 min), and rinsed twice with PBS. Before analysis the cells were suspended in PBS with 0.1% PFA. Phenotypic determination of the white blood cell population was made using FSC/SSC parameters. The results are presented as the mean % ± standard error of the mean.
Biochemical analysis
Total protein, albumin and glucose, and alanine (ALT) and aspartate (AST) transaminase activity were evaluated by enzymatic colorimetric method according to the manufacturer’s protocol (Pointe Specific, Poland). The results are presented as mean ± SEM. All analyses were performed in duplicate.
Results
Body weight and food consumption
Rats fed a control diet (20% of protein, group I) weighed 575.9 ±15.8 γ (mean ±SEM) on the 90th day of experiment. Exercise did not affect the average body weight of rats fed the control diet (20% protein + exercise group II). The rats fed a protein-deficient diet (4.5% protein, group III) had body weight over two times lower (p < 0.001) than the rats fed the control diet. A similar trend was observed in the exercised rats (4.5% protein + exercise, group III p < 0.001). Supplementation of B6 vitamin caused a significant increase of the body weight (about 20-35%, p < 0.01) in unexercised (4.5% of protein + vitamin B6, group V) and exercised rats (4.5% of protein + vitamin B6 + exercise, group VI). Daily food intake was highest in both control groups: unexercised (I) and exercised (II) rats; 26 and 28 g/day, respectively. Protein malnourished rats consumed less food per day: generally, 21-22 g/day regardless of the experimental group.
The average food consumption and the final body weight of the rats fed experimental diets (I-VI) assessed on the 90th day of experiment are shown in Table 1.
Table 1
The effect of a protein-deficient diet non-supplemented or supplemented with vitamin B6 on the average consumption and body weight of exercised or unexercised rats. Rats were fed for 90 days with control diet (20% of energy from protein, groups I and II), low-protein diet (4.5% of energy from protein), group III and IV, or low-protein diet (4.5% of energy from protein) supplemented with 300% of vitamin B6 (group V and VI). a, b, c – the level of divergence between groups (p < 0.05). Each group consisted of 10 animals
Organs and tissues
The average weight of organs and tissues isolated from rats fed experimental diets (I-VI) on the 90th day of experiment are shown in Table 2.
Table 2
The effect of a protein-deficient diet non-supplemented or supplemented with vitamin B6 on selected organs and tissue weights in the exercised and unexercised rats. Rats were fed for 90 days the control diet (20% of energy from protein, groups I and II), low-protein diet (4.5% of energy from protein, groups III and IV), or low-protein diet (4.5% of energy from protein) supplemented with 300% of vitamin B6 (groups V and VI). a, b, c, d – the level of divergence between groups (p < 0.05). Each group consisted of 10 animals
The rats fed the protein-deficient diet had lower body weight and lower mass of all studied organs (p < 0.001). Supplementation with vitamin B6 caused a significant increase (p < 0.05) of kidneys, lungs, heart, testes, and muscle mass. There was no significant difference in the mass of liver and spleen between protein-deficient groups (III-VI). Exercise did not affect organ weight, with the exception of muscle (soleus) mass, which was significantly higher in the 20% protein group (II). This trend was also observed in the protein-deficient groups (IV and VI); however, the differences were not statistically significant.
Haematological analysis
The results of haematological analysis of the control group (I) were typical for the rat. Exercise did not affect the haematological parameters studied in the control (II) or protein-deficient diet (IV and VI) groups. Protein deficiency caused a significant reduction of haemoglobin concentration (about 20%, p < 0.05) and a decrease in the mean corpuscular volume (MCV, about 10%, p < 0.05) and the mean corpuscular haemoglobin (MCH, about 10%, p < 0.05) in both the exercised and unexercised rats. Vitamin B6 supplementation of the protein-deficient groups (V and VI) increased the haemoglobin concentration to the level observed in the control groups (I and II). However, the MCV and MCH parameters were unaffected by the vitamin B6 addition.
The results of haematological blood analyses of the rats fed the control diet (I, II), the protein-deficient diets (III-IV), and the protein-deficient diets supplemented with vitamin B6 (V-VI) are presented in Table 3.
Table 3
The effect of a protein-deficient diet non-supplemented or supplemented with vitamin B6 on haematological parameters in exercised or unexercised rats. Rats were fed for 90 days the control diet (20% of energy from protein, groups I and II), low-protein diet (4.5% of energy from protein, groups III and IV), or low-protein diet (4.5% of energy from protein) additionally supplemented with 300% of vitamin B6 (groups V and VI). a, b, c – the level of divergence between groups (p < 0.05). Each group consisted of 10 animals
Immunological analysis
White blood cell subpopulations
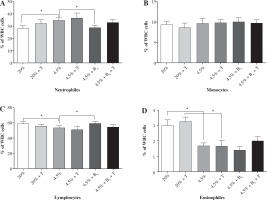
There was no significant difference in the white blood cell subpopulations (neutrophils, eosinophils, monocytes, and lymphocytes) between exercised and unexercised rats in all studied groups (I-VI). The rats fed protein-deficient diets had a higher percentage of neutrophils (about 20%, p < 0.05) and a lower percentage of lymphocytes (about 10%, p < 0.05). Both groups fed the protein-deficient diet (group II-VI) presented the level of eosinophils at half the norm. Supplementing the diet with vitamin B6 reduced the effect of protein malnutrition on the percentage of neutrophils and lymphocytes; it did not, however, affect the level of eosinophils.
The results of the white blood cell subpopulation analyses in the rats fed the control diet (I, II), the protein-deficient diet (III-IV), and the protein-deficient diet supplemented with vitamin B6 (V-VI) are presented in Figure 1.
Fig. 1
The effects of a protein-deficient diet, non-supplemented or supplemented with vitamin B6, on the white blood cell subpopulations in exercised and unexercised rats. The rats were fed the following diets for 90 days: a control diet (20% of energy from protein, groups I and II), low-protein diet (4.5% of energy from protein, groups III and IV), or low-protein diet (4.5% of energy from protein) additionally supplemented with 300% of vitamin B6 (groups V and VI). *– the level of divergence between groups (p < 0.05)
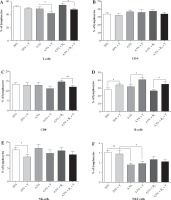
Lymphocyte subpopulations
Determination of lymphocyte subpopulations in the blood of rats fed the control diet showed the percentage distribution typical for the rat [21, 22]. A protein-deficient diet caused a significant reduction of NKT cells in all studied groups (II-IV); the reduction was not affected by vitamin B6 supplementation. There were several significant changes observed in the exercised rats (groups II, IV, and VI) in comparison to the control groups. The percentage of T-cells (CD3+) was lower (about 15%, p < 0.05) in the blood of rats fed the protein-deficient diets (both the non-supplemented and supplemented with vitamin B6). These changes were associated with the reduction in the percentage of CD8+ T cells. This reduction (about 20%) was significant (p < 0.01) in exercised rats fed the protein-deficient diet supplemented with vitamin B6. In contrast to the T-cells, the percentage of B-cells was higher in all exercised rat groups (over 20%, p < 0.05), with the highest difference (about 32%, p < 0.05) in the protein-deficient group supplemented with vitamin B6. There was also a significant decrease (about 30%, p < 0.05) in the percentage of NK cells, but only in the rats fed the control diet.
The blood lymphocyte percentages in rats fed the control diet (I, II), protein-deficient diet (III, IV), and protein-deficient diet supplemented with vitamin B6 (V, VI) are presented in Figure 2.
Fig. 2
The effects of a protein-deficient diet, non-supplemented or supplemented with vitamin B6, on the blood lymphocyte subpopulations in exercised and unexercised rats. The rats were fed the following diets for 90 days: a control diet (20% of energy from protein, groups I and II), low-protein diet (4.5% of energy from protein, groups III and IV), or low-protein diet (4.5% energy from protein) additionally supplemented with 300% of vitamin B6 (groups V and VI). * – the level of divergence between groups (p < 0.05)
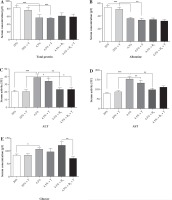
Biochemical analysis
The total protein and albumin concentration in serum was significantly decreased in rats from all protein-deficient groups (II-VI, about 30%, p < 0.001). There was no significant effect of exercise or vitamin B6 supplementation on the concentration of total protein and albumin (Fig. 3). The protein deficient diets significantly elevated the activity of aminotransferases (both alanine and aspartate about 90%, p < 0.001) in unexercised and exercised rats (alanine – 66%, p < 0.01 and aspartate – 53%, p < 0.05). Vitamin B6 supplementation erased the negative effect of protein deficiency on aminotransferase activity in both exercised and unexercised rats (Fig. 3). The unexercised and exercised rats fed the control diet did not differ in concentration of glucose. The rats fed the protein-deficient diet (group III) had a higher concentration of glucose in serum than the rats fed the control diet (27%, p < 0.05). Vitamin B6 supplementation did not affect glucose metabolism in the unexercised rats; however, it significantly reduced glucose concentration in the exercised rats (p < 0.01, Fig. 3).
Fig. 3
The effects of a protein-deficient diet non-supplemented or supplemented with vitamin B6 on the total protein, albumin and glucose concentrations, and aminotransferases activity (ALT and AST) in the serum of exercised and unexercised rats. The rats were fed the following diets for 90 days: a control diet (20% of energy from protein, groups I and II), low-protein diet (4.5% of energy from protein, groups III and IV), or low-protein diet (4.5% of energy from protein) additionally supplemented with 300% of vitamin B6 (groups V and VI). * – the level of divergence between groups (p < 0.05)
Discussion
The general effects of long-term protein deficiency are well-known; however, there is still insufficient knowledge on the effect of protein deficiency combined with prolonged exercise. Here we evaluated the effects of moderate, long-term (90 days) exercise on selected haematological, immunological, and biochemical blood parameters in rats fed a normal diet, a protein-deficient diet, and a protein-deficient diet supplemented (300% of the norm) with vitamin B6.
The body weight of rats (both exercised and unexercised) fed the protein deficient diet for 90 days decreased almost twice as much as in the control rats. This result is concurrent with earlier findings from our and other laboratories [21-24]. Vitamin B6 supplementation significantly affected the body mass of rats fed the protein-deficient diet. However, interestingly, the average food intake was the same for non-supplemented protein-deficient groups (III and IV) and vitamin B6 supplemented groups (V and VI). It is well-known that vitamin B plays an important role in fat synthesis [25]. Vitamin B1 and B6 are required for the synthesis of fat from carbohydrates and proteins. The increase in the body mass observed in our study in the vitamin B6 supplemented groups may therefore be associated with increased synthesis of fat. However, a macroscopic analysis of selected organs did not confirm this hypothesis. All examined organs (liver, kidney, lungs, heart, testes, thymus, spleen, and muscle) in vitamin B6 supplemented groups were heavier in comparison to the protein-deficient groups; however, the weight was proportional to the body mass (Table 2). Moreover, livers from the rats fed protein-deficient diets (groups III and IV) had a higher relative mass ratio (organ weight/rat weight), which was associated with increased activity of aminotransferases in the blood (AST and ALT). Such abnormalities are indicative of liver steatosis. Development of this pathology in the protein-depleted organism was previously shown in many species including the rhesus monkey [26], rat [27], and mouse [28]. Interestingly, vitamin B6 supplementation reduced the negative effect of protein malnutrition on the liver and decreased AST and ALT activity. This suggests that the supplementation of the protein-deficient organism with vitamin B6 improves food utilisation and normalises fatty acid metabolism. This suggestion partially confirms the findings of Dębski et al. [29] on rats fed a protein-deficient diet, where the addition of vitamin B6 to the protein-deficient diet caused a partial reversal of changes observed in the hepatic FA composition. However, vitamin B6 supplementation has a negative side effect – it increases glucose concentration in the blood. We observed a higher concentration of glucose in the serum of unexercised, vitamin B6 supplemented rats. The glucose concentration was reduced to the control level by exercise (group VI). This observation may explain the negative effect of vitamin B6 on obese human subjects suffering from hepatic steatosis. In these patients, there was a positive correlation between hepatic steatosis, the controlled attenuation parameter (CAP) score and vitamin B6/total protein ratio, triglycerides, glucose, alanine aminotransferase (ALT), and body mass index [30]. Our results and the fact that the low-protein diet reduces body mass [31], decreases liver fat deposition, and improves markers of muscle metabolism in obese Zucker rats [32] suggest that these effects of vitamin B6 are associated with the increase of glucose level in the blood.
Regular exercise improves the function of the cardiovascular system and decreases the possibility of heart injury [33]. Here, we did not observe beneficial effects of exercise on the rat weight and blood parameters (Table 1 and 2). We only noted an increase (about 20%) of the average weight of the soleus muscle, which indicated that the exercise procedure was performed correctly. We believe that the lack of difference in the body mass between control groups (exercised and unexercised) was associated with the ad libitum feeding model. This model of nutrition may deliver excessive amounts of calories, and it is known that only a proper supply of calories results in weight loss [34]. Vitamin B6 is involved in proper functioning of the haematological system. Vitamin B6 deficiency caused a reduction of the mean corpuscular volume (MCV), haemoglobin, and haematocrit (HCT) in mouse blood; the parameters were restored after three days of vitamin B6 supplementation [35]. We showed here that the addition of vitamin B6 to the protein-deficient diet improved the haemoglobin concentration in both exercised and unexercised rats, but it did not affect HCT and MCV parameters. This lack of change in HCT and MCV parameters may be associated with a poor protein status and could explain the differences of the results obtained after 60 days of supplementation with vitamin B6 [22]. We also found another positive effect of addition of vitamin B6 to the protein-deficient diet. Vitamin B6 statistically increased the muscle to weight ratio (organ weight/rat weight) in comparison to B6-unsupplemented protein-deficient groups (III p < 0.001 and IV p < 0.009). This may be related to the effect of vitamin B6 on the expression of muscle-specific genes. Suidasari et al. [36] showed that myokines, Nrf2-related factors, myogenin, and HSP60 were upregulated in humans given the recommended dietary intake of vitamin B6. These factors promote the growth and repair of skeletal muscles. Moreover, the level of vitamin B6 positively correlated with the level of carnosine, a histidine-containing dipeptide, which is well known to be associated with the performance of skeletal muscles [37].
Here, we observed a significant reduction of lymphocyte and eosinophil percentages as well as an increase of neutrophil percentage in the rats fed a protein-deficient diet. An increased level of immune cells from the adaptive immunity pool in the blood may indicate a switch from the innate to the adaptive immune response during protein deficiency. Such a switch was also confirmed by Calder and Jackson [38]. We showed herein that the protein deficiency also affected the involution of thymus (a higher relative mass compared to the control group, Table 1), which may result in the delay in development of T cells in the thymus. Addition of vitamin B6 normalised the percentage of lymphocytes and neutrophils in the blood, but had no effect on the eosinophil level. This may indicate that vitamin B6 erases the negative effects of protein deficiency and improves the cooperation between the innate and adaptive immunity.
In humans, there are several characteristic patterns of change in the lymphocyte parameters following long-term physical exercise [39]. Physical activity suppresses the innate response and decreases lymphocyte proliferation. Physical activity recruits all subpopulations of lymphocytes. The number of neutrophils increases during exercise and immediately after, whereas the number of lymphocytes increases during exercise and decreases immediately after. The numbers of CD4 T cells, CD8 T cells, CD19 B cells, CD16 natural killer (NK) cells, and CD56 NK cells increase during exercise and decline after intense exercise lasting at least one hour [40]. In our study the most noticeable effect of exercise was on the number of B cells. In each type of diet, the number of B cells significantly increased after physical activity. Pedersen and Nieman [41] showed that long-term physical activity inhibits the activity of B cells, which in turn may result in their elevated levels in blood. In our study the main difference between the control and the protein-deficient groups was the lower percentage of NKT cells. Similar results were obtained in our previous study [22]. Qian et al. [10] found that vitamin B6 deficiency influenced immunity and Cheng et al. [42] noticed a positive role of vitamin B6 in improving immune responses in critically ill patients. On the other hand, Jankowska et al. [43] observed that the level of vitamin B6 did not correlate with the lymphocyte count, immune response, or the level of inflammation markers in kidney transplant recipients.
Conclusions
Supplementation of vitamin B6 significantly affected health parameters of protein-malnourished rats. It increased body and muscle mass, decreased liver parameters, and normalised the blood haemoglobin concentration and percentage of white blood cell subpopulations. Exercise did not affect the tested parameters, with the exception of glucose concentration, which was elevated in the unexercised rats and reduced in exercised rats. The results of this study indicate that vitamin B6 should be considered a useful supplement for individuals suffering from protein malnutrition.


