Introduction
Vitiligo and insulin resistance have been known for many years. Descriptions of vitiligo lesions date back to ancient times [1], while insulin resistance was observed and recognized in the last century [2]. In recent years, there have been significant developments in research to trace and understand the aetiology of both conditions.
The purpose of this article is to review insulin resistance (as a component of metabolic syndrome) and vitiligo, focus on the pathophysiological mechanisms, and analyse clinical studies evaluating the association of these two disorders.
Vitiligo
Vitiligo is the most common disease causing skin depigmentation (0.5–2% of the population worldwide) [3]. It is an acquired, chronic autoimmune disorder associated with a selective loss of melanocytes and consequent hypopigmentation in the affected areas of the skin [4]. Clinically, it manifests as a chalky-white patch with distinct edges. Recent findings and evidence-based medicine suggest a link between this disease and genetic [5, 6] and environmental factors, oxidative stress, inflammatory mediators, and disorders of cellular detachment. There are also neurohumoral hypotheses related to the involvement in the pathogenesis of this disorder [7]. Vitiligo can be divided into segmental vitiligo (SV) and non-segmental vitiligo (NSV) [4]. Since 2011 SV has been classified as a separate disease entity and the term vitiligo refers to the remaining forms of NSV [3]. SV differs from NSV in its unilateral and segmental distribution, early melanocyte involvement, early age of onset, and rapid stabilization, whereas in NSV vitiligo lesions are usually bilaterally distributed symmetrically throughout the body and evolve over time [4]. SV tends to have an earlier onset and a rapidly progressive but limited course. Depigmentation in SV spreads within the segment over a period of 6–24 months, and then stops. In addition, SV is characterized by early involvement of hair follicle melanocytes, and some patients show polyposis in the affected areas. Furthermore, melanocyte autografting usually yields good results for SV with persistent pigmentation. Therefore, it is agreed that the term vitiligo (V) in this article is the recommended term for all non-segmental forms of vitiligo, while segmental vitiligo refers to a clinically unambiguous segmental distribution of depigmented lesions [8].
It is therefore agreed that the term vitiligo (V) is the recommended term for all non-segmental forms of vitiligo, while segmental vitiligo refers to a clinically unambiguous segmental distribution of depigmented lesions, usually associated with rapid onset and leucotrichia. NSV includes acrofacial, mucosal, generalized, universal, mixed, and rare variants. The pathophysiology of vitiligo is very complicated and many mechanisms are still undiscovered, which is why the pathophysiology of vitiligo is still the subject of research and analysis today. Studies report that oxidative stress may have a key role in initiating melanocyte degradation, which in response to stress produces reactive oxygen species (ROS) that destabilize the balance between oxidative stress markers and antioxidants [9] and cellular metabolism [10]. Melanogenesis itself is a process that generates a pro-oxidant state, so ROS in vitiligo come from both extracellular processes and those that occur intracellularly. Oxidative stress implies a number of changes in the melanocyte, ranging from mitochondrial function to cellular proteins, cation channels, and membrane lipids, as well as receptors on the cell membrane surface. These changes ultimately lead to melanocyte apoptosis [10]. Reduced expression of cadherins in altered skin caused by oxidative stress results in reduced melanocyte adhesion, which we can see clinically as the Koebner phenomenon [11]. Natural killer (NK) cells have been shown to be involved in the early response [12] to oxidative stress in vitiligo patients [13]. It is also believed that inducible heat shock protein 70 is involved in the pathogenesis of vitiligo, which consequently affects the destruction of melanocytes by T lymphocytes (CD8+) [10]. The number of these lymphocytes in blood in patients with vitiligo is higher and correlates with disease activity [14]. The diagnosis of the disease is based on the clinical picture of the lesions’ typical distribution: around the mouth, lips, distal limbs, penis, segmentally, and friction sites [7]. Diagnosis may be further facilitated by examination of the skin with a Wood’s lamp, which highlights areas of depigmentation that may not be visible to the naked eye. The Assessment Form created by the Vitiligo European Task Force may be useful in evaluating a patient with vitiligo; it summarizes elements of the personal and family history and elements of the clinical examination. Clinical markers of disease activity may include Koebner’s phenomenon, trichrome changes, inflammatory changes, and confetti-like depigmentation, keeping in mind also the overall assessment of the patient’s mental status and quality of life [3]. There are many methods to assess vitiligo activity, but so far there has been no standardized method. Among them, there are two scales to assess disease activity and response to treatment. These are the VIDA scale, which is a subjective method of assessment, and the VASI scale, which is a semi-objective method [15]. Patients with vitiligo are more likely to have not only other autoimmune diseases [11, 16] but also other diseases [17].
Insulin resistance as a component of metabolic syndrome
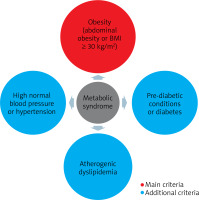
The term insulin resistance means nothing more than the inability of a known amount of exogenous or endogenous insulin to increase glucose uptake and utilization to the same extent as in a healthy person [18]. The essence of insulin resistance is the effect of insulin on glucose uptake and utilization and the inhibition of hepatic glucose production [2]. It is an undeniable fact that insulin resistance (mainly as a component of metabolic syndrome) underlies the development of many different and complex disease entities and syndromes, in the pathogenesis of which genetics plays a role in addition to insulin resistance [2, 19]. The pathogenesis of insulin resistance is also complex and multifactorial. There are many mechanisms of its formation, among which both environmental and genetic factors play a role. In insulin resistance syndrome, insulin function related to glucose uptake, inhibition of adipose tissue lipolysis and vasodilation are impaired [2]. The cutaneous manifestation of this process may be Acanthosis nigricans, which is associated with hyperinsulinemia and obesity and may serve as a marker of insulin resistance [20]. The most common and widely used tool to assess insulin resistance is the HOMA-IR = (FPI × FPG)/22.5, in which FPI is the fasting plasma insulin concentration (mU/l) and FPG is the fasting plasma glucose concentration (mmol/l) [21, 22]. There is also another way of calculating insulin sensitivity that relies less on insulin concentration and is called the quantitative insulin sensitivity control index (QUICKI) [23]. A better correlation of QUICKI with the euglycemic buckle than HOMA-IR and a lower coefficient of variation were observed [18]. It is concluded that insulin resistance as a component of the metabolic syndrome is implicated in autoimmune skin diseases, and chronic inflammation may explain the relationship between one condition and the other [24]. Metabolic syndrome has been extensively researched and, according to the 2022 definition [25], which takes into account the progress that has been made in understanding its various components, the presence of obesity and two of the three: elevated blood pressure, abnormal glucose metabolism or elevated non-HDL fraction cholesterol. In addition, the new definition recommends paying attention also to its other additional components such as impaired renal function, hepatic steatosis, obstructive sleep apnoea (OBS), heart failure with preserved ejection fraction, polycystic ovary syndrome (PCOS), chronic inflammation, sympathetic nervous system activation and hyperuricemia (Figure 1).
The association between metabolic syndrome (and insulin resistance as its component) and vitiligo: a research overview
There have been many studies on the association between vitiligo and metabolic syndrome [26, 27], while there have been few scientific papers on vitiligo and insulin resistance that have considered the HOMA-IR index. A study by Karadag et al. showed an association between vitiligo and IR, and patients in the study group had higher levels of HOMA-IR, insulin, and C-peptide than those in the control group [28]. Also Ibrahim et al. [29] conducted a case-control study in which participants were assessed for metabolic syndrome using the International Diabetes Federation (IDF) criteria in addition to insulin resistance via homeostasis model assessment of IR (HOMA-IR). Their results showed a significantly more frequent association with high fasting plasma glucose levels, high blood pressure readings, central obesity, dyslipidaemia, and metabolic syndrome than controls (p = 0.020, p = 0.034, p = 0.014, p < 0.001, and p = 0.002, respectively) and also significantly higher levels of fasting insulin and HOMA-IR (p ≤ 0.001).
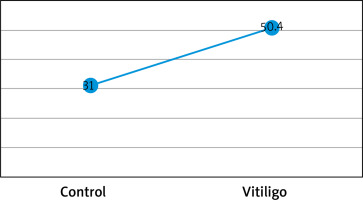
Figures based on the two authors’ studies show parameters describing patients with insulin resistance and vitiligo compared to a control group (without vitiligo) (Figures 2, 3).
Figure 2
Insulin resistance parameters in vitiligo patients based on the study by Karadag et al. [28]
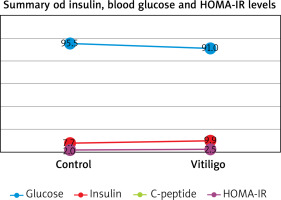
Figure 3
Percentage (%) of insulin resistance in vitiligo patients and controls based on the HOMA-IR index from the study by Ibrahim et al. [29]
Discussion
Metabolic syndrome
The relationship between metabolic syndrome and vitiligo has been widely studied and described in various studies, which cite oxidative stress, through the production of ROS, as components of the pathogenesis of both conditions. ROS disrupt mitochondrial function by which they have a role in the development of metabolic syndrome due to defective cellular metabolism. In turn, increased glucose levels intensify the overproduction of ROS leading to morphological changes in mitochondria [30–32]. Accumulation of lipids and free fatty acids results in inhibition of the insulin signalling pathway, which contributes significantly to the development of metabolic syndrome [32]. There are also mentioned pro-inflammatory cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, IL-1-β, IFN-γ found in patients with active vitiligo, and some anti-inflammatory cytokines such as IL-5 and IL-10 [33]. In the pathogenesis the following are also involved catecholamines and homocysteine, the cause of hypertension [26] – one of the components of the metabolic syndrome, elevated levels of which are found in patients with vitiligo [34–36]. Studies have shown an increased risk of developing metabolic syndrome in patients with vitiligo and have considered clinical features of the disease, such as active, extended and segmental vitiligo of extended duration as independent factors in the development of metabolic syndrome [37, 38]. In contrast, another study showed that the incidence of metabolic syndrome is increased with the severity/activity of vitiligo [39]. Considering the division of vitiligo into segmental and non-segmental, segmental vitiligo has been shown to be mainly associated with genetic and autoimmune factors [7, 40], while metabolic syndrome is more common in patients with non-segmental vitiligo [38, 39, 41].
Insulin resistance
Both insulin resistance and vitiligo have an assumed and multiple pathogenesis. It has been proven that vitiligo is characterized by the involvement of this disease not only in the skin, but the disease also shows systemic interference [17]. There are studies and hypotheses about the common mechanisms of both these disorders. In addition to glucose transport, insulin performs a number of other functions in the body [42].
Insulin is a regulator of keratinocyte proliferation and differentiation, which is a prerequisite for the formation of epidermal structure [18]. One hypothesis involves mechanisms associated with elevated levels of pro-inflammatory cytokines in patients with vitiligo. Under conditions of chronic inflammation [43], high concentrations of pro-inflammatory cytokines are involved in the inhibition of the insulin signalling pathway through activation of p38MAPK and consequent phosphorylation of serine residues of the insulin-1 receptor substrate, leading to the development of insulin resistance in vitiligo [28, 44–47]. Cytokines such as TNF-α, IL-1, IL-6 and C-reactive protein (CRP) have been shown to contribute to the induction of insulin resistance and other metabolic complications and cardiovascular disease [44–46, 48]. The second theory involves MCHR autoantibodies, which have been shown to induce damage to human melanocytes in vitro through antibody-dependent cytotoxicity [49]. In contrast, MCH and MCHR1 have been detected in primary human islets, suggesting a potential role of this neurotransmitter in modifying islet function and stimulating insulin secretion via MCH [50]. Therefore, there may be an association between MCHR1 autoantibodies present in vitiligo patients and hyperinsulinemia and, consequently, IR [51]. The association of elevated IR in patients with vitiligo may also be explained by the presence of shared susceptibility loci between diabetes and vitiligo [27, 52, 53].
There is a need for continued research and experimentation to elucidate the pathophysiological processes involved. Also, there are too few clinical studies that take into account the HOMA-IR index in vitiligo patients, which can provide additional contribution to research [38]. Analysis of the clinical picture of vitiligo lesions in relation to the severity of insulin resistance should also be considered. There are few clinical studies, which would take into account, apart from HOMA-IR index, tools for assessment of vitiligo severity [29] (e.g. VIDA and VASI scales). Also, there are little data on the location of vitiligo lesions in patients with insulin resistance.
Conclusions
There is a need for more knowledge on the pathogenesis of vitiligo and insulin resistance. It is also worth considering the clinical picture of vitiligo lesions in patients with insulin resistance. Few studies, circumstantial evidence and conjectures lead to concluding that the subject of vitiligo and insulin resistance still remains a broad field for analysis and research.