Introduction
Malignant melanoma (MM) is a highly aggressive skin tumour that exhibits a wide range of cellular morphological variability. The disease has a high rate of metastasis and recurrence, and is the most serious type of skin cancer, causing the vast majority of deaths. The number of deaths due to MM accounts for about 1% of all skin cancer cases [1]. MM is not only found in skin-related cancers, but also related to the occurrence of retinoblastoma, meningioma, breast cancer, and colorectal cancer [2, 3]. It is reported that malignant anorectal melanoma is an extremely rare cause of rectal tumours, accounting for less than 1% of all melanomas and about 4% of all malignant colorectal tumours [4]. Although the incidence of MM is low in the occurrence of cancer, its development rate is the fastest. At present, the treatment methods of MM include surgical resection, chemotherapy, immunotherapy, targeted therapy, radiotherapy, and high-temperature inactivation therapy [5, 6]. Among them, targeted therapy is more effective for patients with advanced MM cancer. Studies have found that miRNAs play an important regulatory role in the occurrence and development of melanoma and can be used as biomarkers in the diagnosis of melanoma [7].
miRNAs are a class of non-coding RNA molecules with a length of about 22 nucleotides, which can regulate gene expression by binding to the 3’ untranslated region of target genes and leading to the degradation of target genes or inhibiting the transcription process. Studies have confirmed that miRNA can act as a tumour suppressor or tumour inducer. As against the physiological state of normal tissues, the expression profile of miRNA in cancer cells shows abnormal changes. Based on the effect of miRNA on its target genes, it has been confirmed that miRNA can inhibit or promote tumour growth [8]. The increase of oncogenic miRNA is caused by the abnormal amplification and ectopic of miRNA genes. The deletion, mutation, and promoter methylation of miRNA affect the development of malignant melanoma. In the treatment of melanoma with anti-programmed cell death protein 1 (PD-1) immunotherapy, the up-regulation of miR-21-3p and miR-671-5p has been found to have a positive effect on the treatment of melanoma [9, 10]. Studies have also confirmed that the down-regulation of miR-5100 can inhibit the metastasis of melanoma cells (MCs) [11]. Some studies used MCs (WM-266-4) as research material and the results suggested that the expression of miR-22-3p in the melanoma group was lower relative to the controls, and miR-22-3p suppressed the epithelial-mesenchymal transition (EMT) of MCs by regulating recombinant human galectin-1 (LGALS1) [12].
NOD-like receptor protein 3 (NLRP3) plays a key role in inflammation and immune response, and belongs to the NOD-like receptor (NLR) family. It is a kind of receptor protein that can sense abnormalities in the internal and external environment and activate the immune response [13]. When cells are injured, infected, or abnormally stimulated, NLRP3 protein is activated and forms a complex called the inflammasome [14]. Inflammasome can activate cysteinyl aspartate specific proteinase-1 (Caspase-1), and then trigger the production and release of interleukin-1β (IL-1β) and IL-18, etc., leading to the occurrence of inflammatory response. Studies have found that NLRP3 plays a pathogenic role by regulating innate and adaptive immunity as well as cell apoptosis and differentiation in tumours. The expression of NLRP3 is increased in many tumour tissues, and the overexpression of NLRP3 in liver hepatocellular carcinoma (LIHC) and ovarian cancer (OV) reduces the overall survival (OS) of patients. By examining the relationship between NLRP3 overexpression and immune checkpoints, it was found that NLRP3 can promote the immune escape of cancer cells [15]. Therefore, the expression of NLRP3 can be reduced by suppressing the expression of immune checkpoints, thereby promoting the killing effect of T cells on cancer cells. Some studies have also found that NLRP3 is related to tumour cell invasion and migration. Zhang et al. [16] found that activation of NLRP3 inflammasome pathway can promote gastric cancer cells. Some studies have also found that NLRP3 is related to the treatment of breast cancer, and the treatment of breast cancer can be improved by manipulating NLRP3 inflammasome through miRNA [17]. Thus, NLRP3 can both positively and negatively regulate the treatment of cancer. Studies have shown that the expression of NLRP3 and IL-1β is higher in cutaneous melanoma samples as against normal skin, and the activation of NLRP3 in MCs is a pro-tumour mechanism that can induce the expansion and immune evasion of myeloid-derived suppressor cells (MDSCs) [18]. Malignant melanoma is still one of the worst outcome cancers in the world, and our current knowledge about the role of miRNAs is emerging. While the role of miRNAs is evaluated in many cancers as well as the melanoma, still there are so many inflammatory factors that are affective in this cancer progression. So, based on the fundamental hypothetical knowledge drawn by the literature review, in this study, the human MCs line (WM239a) and human epidermal melanocytes (HEM) were selected as study material, and RT-qPCR, Western blot (WB), cell counting kit-8 (CCK-8), Transwell cell invasion assay, and scratch assay were used.
Aim
This study aimed to investigate whether miR-22 is involved in the activities of MCs by negatively regulating NLRP3 gene.
Material and methods
Research material
Human MCs WM239a (product number: HTX1839C, purchased from Shanghai Hongshun Biotechnology Co., LTD.) was applied, and HEM (product number: 104-05A, purchased from Sigma) were used as controls.
Cell culture
MCs WM239 and normal human melanocyte HEM were cultured in RPMI-1640 medium supplemented with 10% FBS. The culture conditions were 37℃ and 5% CO2 saturated humidity. The medium was changed once a day to maintain the healthy growth of the cells. When the cells reached 60% to 70% confluence, they were digested and passaged using trypsin to maintain cell proliferation and expansion. During the first three passages, it was remembered to cryopreserve the seed. In the experiment, cells in the log phase were selected for operation to ensure the accuracy and reliability of the experimental results.
Cell transfection and grouping
When WM239 was cultured to 80%, 0.25% trypsin was used to digest and collect the cells, and then the cells were reseeded in the plate with 96 wells for culture. When the cell proliferation reached 80%, the liquid in the plate was changed, the serum-free RPMI-1640 medium was adopted for culture for 12 h, and then transfection was carried out. miR-NC, miR-22, anti-miR-NC, and anti-miR-22 were transfected into WM239, which were grouped. miR-22 + pcDNA3.1 empty vector, miR-22+ pcDNA3.1-NLRP3 were transfected into WM239, respectively, which were grouped. Transfection experiments were carried out adopting Lipofectamine 2000 reagent, which was performed strictly according to the instructions.
Detection of cell proliferation activity
CCK-8 method was adopted to detect the cell activity of miR-NC, miR-22, miR-22 + pcDNA3.1, and miR-22 + pcDNA3.1-NLRP3 groups. When the cells were cultured for 24 h, 48 h, and 72 h after transfection, 10 µl CCK-8 solution was added to each well, and the mixture was fully mixed by shaking. The plate with 96 wells was then placed in an incubator, and 4 h later, the plate was placed at 450 nm in a microplate reader for detection.
Note: A(Dosing): OD value of wells with cells, CCK-8 solution, and drug solution; A(0): OD value of wells with cells, CCK-8 solution but no drug solution; A(Blank): OD value of wells without cells.
Cell apoptosis detection
The apoptotic rate was detected by terminal deoxynucleotidyl transferase-mediated dUTP in situ nick end labelling (TUNEL) method. After transfection, 20 µg/ml proteinase K was put to the cultured cells at 24 h, 48 h, and 72 h. After 30 min of full reaction, the medium was removed and PBS buffer was put to wash. Equilibration buffer and terminal deoxynucleotidyl transferase (TdT) were put, incubation in the dark for 1 h. Then, saline sodium citrate (SSC) solution was put, and the cells were washed three times. Then, 4’,6-diamidino-2-phenylindole (DAPI) was put for counterstaining. It was washed after incubation in the dark, and the slides were sealed. The nuclei of apoptotic cells appeared green, and the nuclei of viable cells appeared blue. The sealed slices were observed and the apoptotic rate of cells was calculated.
Cell invasion assay
The Transwell assay was adopted to detect cell invasion. The cells in the four groups were collected, and they were digested with 0.25% trypsin to separate them from the dish wall. Then, serum-free RPMI-1640 medium was put, and the cells were resuspended, and then the cell concentration was adjusted to 2 × 104/ml for cell invasion assay. Cell invasion assay: the Transwell plate was placed into the dish, and Matrigel diluted 5 times with RPMI-1640 medium was put to the filter membrane of the plate, about 40 µl per well. 200 µl of cell suspension of 2 × 104/ml was added to the upper chamber of the Transwell plate. Then, the Transwell chamber was incubated at the appropriate temperature (usually 37°C) and CO2 concentration for 24 h. After the Transwell plate was removed, the cells on the upper surface of the filter membrane were wiped off with a wet cotton swab, and those on the lower surface of the filter membrane were fixed with 4% paraformaldehyde. They were observed by crystal violet staining. The number of cells stained with crystal violet was observed under a microscope.
Cell migration assay
The scratch assay was adopted to detect the migration ability of cells. The cells in the four groups were digested by trypsin, resuspended in RPMI-1640 medium, and seeded in 6-well plates. A straight line of the same width and length was drawn on the bottom of the plate with the tip of a 1 ml pipetting gun. The plate was gently washed with phosphate buffer solution (PBS) to remove free cells, and the scratch area was observed under a microscope after 24 h of culture.
Expression of miR-22 and NLRP3 mRNA
The cells were collected and fully ground to release the total RNA. The total RNA was extracted using Trizol reagent, and the purity and concentration of RNA were determined by ultraviolet spectrophotometer. Then, the RNA was reverse transcribed into cDNA, and miR-22 and NLRP3 mRNA in the four groups were detected by qRT-PCR kit. The reaction system was prepared according to the instructions, and β-actin was adopted as an internal control. The reaction parameters were denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s, and 40 cycles were set up, followed by extension at 72°C for 5 min. Relative expression was computed adopting the 2–ΔΔCt method.
Protein expression measured by WB
miR-NC, miR-22, anti-miR-NC, anti-miR-22, miR-22 + pcDNA3.1, and miR-22 + pcDNA3.1-NLRP3 cells were collected in Eppendorf (EP) tubes and placed on ice boxes. Tissue lysis solution (RIPA) was put to mix thoroughly, and the cells were lysed. Centrifugation (12,000 r/10 min) was then performed, and the supernatant was collected for determination of protein concentration by BCA assay. Then, the protein samples were subjected to SDS-PAGE gel electrophoresis, blocked with 5% skim milk powder after membrane transfer, addition of the primary antibody, incubated at 4°C overnight, addition of the secondary antibody, incubation at room temperature for 2 h, and washing membrane for development. The absorbance of the bands was detected using Quantity One gel analysis software, and the ratio of the absorbance of the target band to GAPDH was adopted to represent the protein expression.
Results
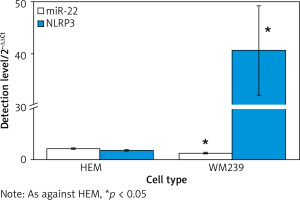
miR-22 expression in WM239 and HEM
Figure 1 illustrates that the expression of miR-22 was 2.15 ±0.13 in human normal melanocyte HEM and 1.24 ±0.09 in human MCs WM239. It was clearly lower in WM239 as against HEM (p < 0.05).
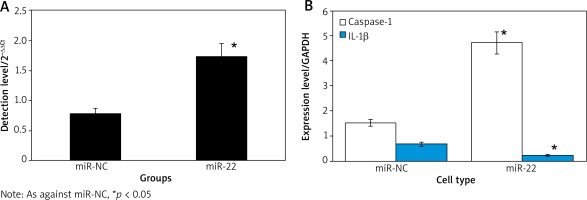
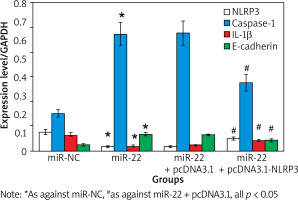
Effect of upregulation of miR-22 expression on WM239 miR-22
Figure 2 displays that the expression of miR-22 and Caspase-1 was clearly higher, and the expression of IL-1β was clearly lower in miR-22 objects as against miR-NC objects (p < 0.05).
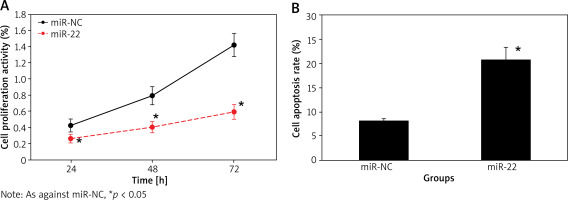
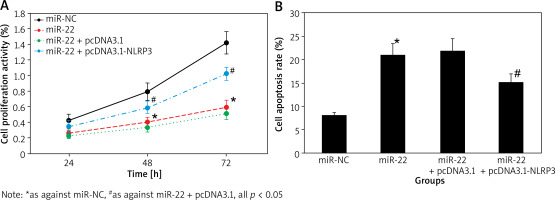
Effect of up-regulation of miR-22 expression on proliferation and apoptosis of WM239
The cell proliferation activity of miR-NC and miR-22 objects at 24 h, 48 h and 72 h suggested an upward trend with the extension of culture time, and that of miR-22 objects was clearly lower as against miR-NC objects. The apoptotic rate of miR-22 objects was clearly higher as against miR-NC objects (all p < 0.05) (Figure 3).
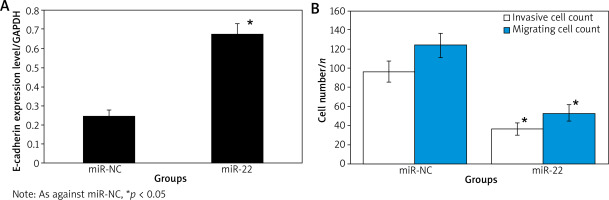
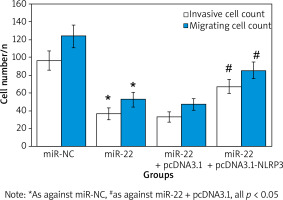
Effect of up-regulation of miR-22 expression on invasion and migration of WM239
WB results suggested that the expression of E-cadherin protein in miR-22 WM239 was clearly higher relative to miR-NC objects. The WM239 invasion and migration assay suggested that the number of WM239 invasion in miR-22 objects (52.67 ±8.58) was clearly lower relative to miR-NC objects (123.64 ±12.75). The number of WM239 migration in the miR-22 objects (36.73 ±6.72) was clearly lower relative to the miR-NC objects (96.63 ±10.64) (all p < 0.05) (Figure 4).
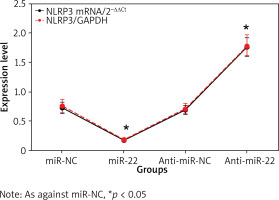
Impact of miR-22 expression on NLRP3
The NLRP3 mRNA in miR-22 WM239 was clearly lower relative to miR-NC objects, and that was apparently higher in anti-miR-22 WM239 relative to anti-miR-NC objects. The expression of NLRP3 protein in miR-22 WM239 was apparently lower as against miR-NC object, and that was apparently higher in anti-miR-22 WM239 as against anti-miR-NC objects (all p < 0.05) (Figure 5).
Effects of up-regulation of NLRP3 expression on WM239
Figure 6 illustrates that the expression of WM239 NLRP3 and IL-1β was apparently inferior, and the levels of WM239 Caspase-1 and E-cadherin were apparently higher in the miR-22 objects as against the miR-NC objects (all p < 0.05).
The levels of NLRP3 and IL-1β were apparently higher, and the levels of Caspase-1 and E-cadherin were apparently inferior in miR-22 + pcDNA3.1-NLRP3 objects as against miR-22-pcDNA3.1 objects (p < 0.05).
Figure 7 A displays that the proliferation activity of miR-22 objects was apparently lower as against miR-NC objects. The activity was apparently higher in miR-22 + pcDNA3.1-NLRP3 objects as against miR-22-pcDNA3.1 objects (all p < 0.05).
Figure 7 B displays that the apoptotic rate of miR-22 objects was apparently higher as against miR-NC objects. The rate was apparently lower in miR-22 + pcDNA3.1-NLRP3 objects as against miR-22-pcDNA3.1 objects (all p < 0.05).
The number of WM239 invasion in miR-22 (36.73 ±6.72) was apparently inferior as against miR-NC (96.63 ±10.64). The number of cell invasion in miR-22 + pcDNA3.1-NLRP3 objects (67.28 ±8.16) was markedly superior as against miR-22-pcDNA3.1 objects (33.46 ±5.83) (all p < 0.05).
The number of WM239 migration in miR-22 (52.67 ±8.58) was markedly inferior as against miR-NC (123.64 ±12.75). The number of cell invasion in miR-22 + pcDNA3.1-NLRP3 objects (85.27 ±9.53) was markedly superior as against miR-22-pcDNA3.1 objects (47.52 ±6.87) (all p < 0.05) (Figure 8).
Discussion
Melanoma is a malignant tumour that is difficult to treat, the disease lacks specific targets, and melanoma develops rapidly and is prone to metastasis [19]. Due to the malignant degree and complexity of melanoma, there is no effective method that can completely cure the disease. Traditional treatment modalities have limited efficacy in the treatment of advanced melanoma. Therefore, it is very important to find new therapeutic targets and strategies for improving the survival rate and treatment outcome of melanoma.
NLRP3 (NLR family, pyrin domain-containing 3) has become an important target for cancer treatment, especially in melanoma cancer. Some studies have found that NLRP3 inflammasome may be a therapeutic target for cutaneous melanoma (CM) and uveal melanoma (UM) [20]. NLRP3 is involved in the regulation of inflammatory response and apoptosis. Sphingosine 1-phosphate (S1P) can interact with S1P receptor 1 (S1PR1) to activate C3 complement processing. After activation of this pathway, intracellular C3b-α’2 interacts with PPIL1 through glutamate 156 (E156) and aspartate 111 (D111) residues, and induce the expression of NLRP3 inflammasome. It has been found that the activation of NLRP3 inflammasome is associated with human metastatic melanoma tissues [21]. It has also been found that rosmarinic acid (RA) can reduce the expression of NLRP3 inflammasome in human MM cells, suggesting that NLRP3 gene can be adopted as a target for the treatment of human MM [22]. The results suggested that the expression level of miR-22 in MCs WM239 was markedly decreased, while the expression level of NLRP3 was increased. When the expression of NLRP3 was up-regulated, the activity ability of MM cells was markedly increased, and the apoptotic rate was decreased. Therefore, suppression of NLRP3 activity can be a potential strategy for the treatment of melanoma and suppress the development of tumours by interfering with the biological characteristics of MCs. EMT is a process by which epithelial tumour cells acquire mesenchymal phenotypic properties, which contributes to cancer metastasis. In EMT, cells change from an epithelial to a mesenchymal phenotype, and enhance the invasion and migration ability of MCs [23]. EMT is related to the occurrence of inflammation, and is also related to tumour invasiveness and poor outcome. Cell heterogeneity is formed by EMT, inflammation and tumour microenvironment, which has a great meaning in the progression, complexity, and chemoresistance of cancer [24]. E-cadherin is related to the adhesion force of cells, and the decrease of its expression level leads to the decrease of cell adhesion force, so that cells acquire the characteristics of easy invasion and metastasis [25]. In this article, it was found that the level of WM239E-cadherin in the miR-22 objects was markedly higher as against the miR-NC objects, and E-cadherin was decreased after up-regulation of NLRP3 expression, suggesting that over-activated NLRP3 can induce EMT.
As a negative regulator, miR-22 plays a major regulatory role in the expression of NLRP3 in melanoma cancer. The results of this article show that downregulation of miR-22 is associated with increased malignant features of MCs. Some studies have proposed that miR-22 can directly regulate the expression of NLRP3 [26], suggesting that miR-22 in this article reduced the expression of NLRP3 by directly targeting the mRNA of the NLRP3 gene. Some studies have shown that MiR-22 is involved in controlling the secretion of proinflammatory cytokines mediated by the NLRP3/CASP1 inflammasome pathway by targeting NLRP3 and HIF-1α. Inflammation is one of the characteristics of cancer, and new therapeutic targets related to the inflammatory tumour microenvironment are currently the focus of exploration [27]. In this article, miR-22 markedly reduced the expression of IL-1β by targeting NLRP3, suggesting that miR-22/NLRP3 may be a therapeutic target related to inflammation.
Sulaiman et al. also conducted a similar study, and their results showed that miR-22 may target and inhibit the activation of NLRP3 inflammasomes, while reducing the activity of A375 cells in skin maleit [28]. Up-regulation of miR-22 has been associated with reduced melanoma cell proliferation, invasion, and migration abilities. This occurs through the negative regulation of NLRP3, impacting downstream factors such as interleukin-1β (IL-1β) and apoptotic rates [29]. The interplay between miR-22 and NLRP3 presents a potential therapeutic target for melanoma treatment, with implications for improving prognosis and treatment outcomes. In clinical context, other investigations confirm the formation of the NLRP3 inflammasome in metastatic melanoma biopsies, emphasizing its relevance in melanoma progression [30].
Conclusions
Downregulation of miR-22 is associated with NLRP3 hyperactivation as well as increased malignant features of MCs. The miR-22/NLRP3 signalling pathway could be a significant focus for future melanoma treatment research. This study concentrates on miR-22, but melanoma progression involves intricate molecular networks of different pathways. While these experiments offer these results, the findings may not fully represent the complex interactions within the human body. Also, this study focused on a specific melanoma cell line (WM239a), and results may not be universally applicable to all melanoma subtypes or different cell lines. Clinical research studies might help targeting this pathway to offer potential therapeutic strategies for melanoma treatment.